- OBJECTIVE:
- To lay down a procedure for Operation of Polarimeter. Make: – Rudolph Research Analytica
- SCOPE:
This SOP is applicable to the procedure for Operation of Polarimeter. Make: – Rudolph Research Analytical at {Company Name} {Location}.
- RESPONSIBILITY:
- Officer/Executive/Designee Quality Control – Shall be responsible for operation as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Polarimeter: A polarimeter is a scientific instrument used to measure the optical rotation of light, which is the angle by which polarized light is rotated as it passes through an optically active substance.
- Check the cleanliness of the work area and instrument.
- Connect the power cord to the electrical supply source.
- Sample compartment shall be kept empty while switching on instrument.
- Switch on the instrument by using the ON/OFF switch present on the backside of the instrument.
- Keep the instrument ON condition at least for10 minutes before operation and wait until the reading on the display come to stable position.
- Press the Zero key and wait until the display shows 0.00 reading.
- Press the wavelength soft key and select the desired wavelength.
- Press the Scale key and select the desired measurement scale like Optical rotation, Specific optical rotation, Specific optical plus or concentration.
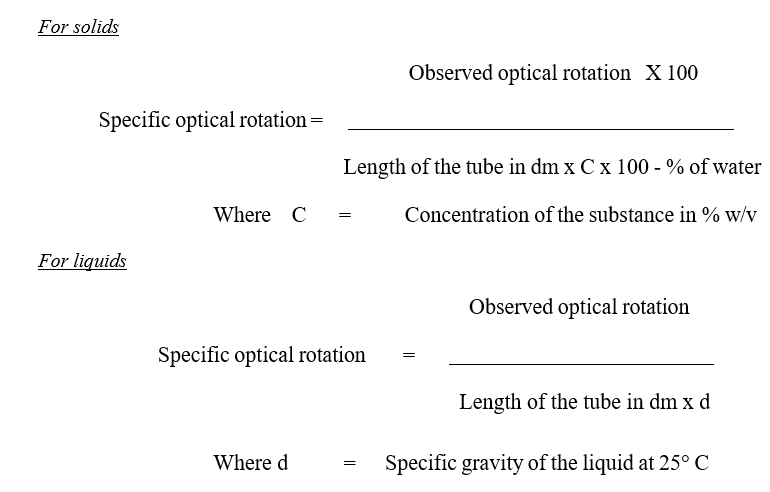
- To measure the optical rotation and specific optical rotation, Cell length and concentration shall be entered.
- Press the Temp control soft key and select the temperature 20°C or 25°C, if different temperature is required then press Input soft key and enter the required temperature (between 15°C to 40°C), if the temperature is not required then press Off.
- Sample cell shall be rinsed with distilled water or solvent in which the sample shall be prepared and fill the same solvent in the sample cell without any air bubble.
- Sample cell shall be placed in the compartment and close the door and observe the reading. Press zero key after the stable reading on the display for blank correction.
- Sample solution shall be poured into the cell and rinse it thoroughly.
- Fill the sample solution into the cell and remove air bubbles if any. Place the cell in the sample compartment and insert the built in temperature probe into cell and close the door.
- Press the Start key to measure the sample, enter operators name, password and press login key.
- Enter the Lot ID and press set.
- Enter the Sample ID and press set.
- The sample optical rotation measurement takes place once the measurement is complete the results are displayed.
- Press measurement data soft key and press 21 CFR 11 data and select report.
- Press the Print key and the report is printed.
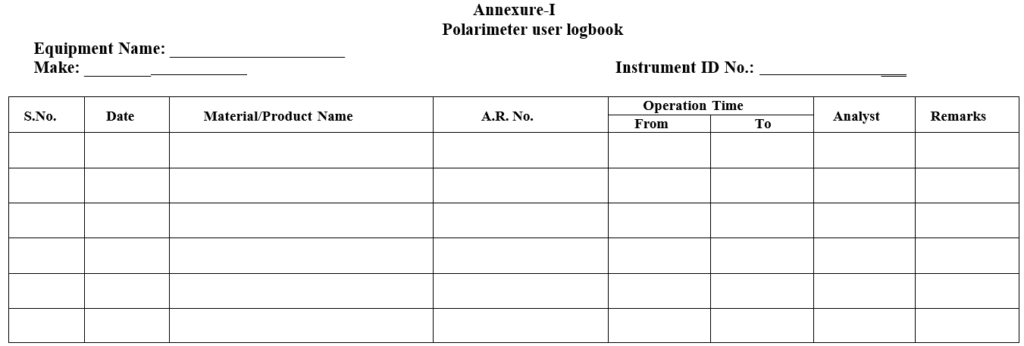
Enter the details of the usage in the User logbook.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Polarimeter user logbook |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| °C | : | Degree centigrade |
| ID | : | Identification |
| SOP | : | Standard Operating Procedure |
| dm | : | Decimeter |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Polarimeter user logbook