- OBJECTIVE:
- To lay down the procedure for calibration of Dissolution test apparatus.
- SCOPE:
This SOP is applicable to the procedure for calibration of Dissolution test apparatus at {Company Name} {Location}.
- RESPONSIBILITY:
- Officer/Executive/Designee Quality Control – Shall be responsible for operation as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Instrument preset up:
- Ensure the working area and apparatus is clean.
- Operate the instrument as per the SOP.
- Fill the water bath with purified water and set the temperature of water bath to 37 ± 0.5°C.
- Install the specified apparatus (i.e.) paddle/basket into the instrument.
- Fill the Specified volume of purified water in all dissolution jars.
- Allow the instrument to stand till the specified temperature is obtained in the jars.
- Instrument calibration procedure:
- The following parameters of the instrument shall be calibrated as mentioned below.
- The physical parameters of instruments shall be calibrated by using validation kit.
- Instrument preset up:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-test-apparatus/
- Jar temperature Calibration by thermometer
- Rotation Per Minute by using Tachometer
- Distance between the bottom edge of basket/paddle to the lowest inner surface of vessel by using depth gauge
- Paddle/basket wobble test by Wobble meter.
- Centering test by Vernier Caliper.
- Calibration of Timer by using Stop Clock.
- Vibration Check
- Apparatus Suitability Test by dissolution calibrator tablets.
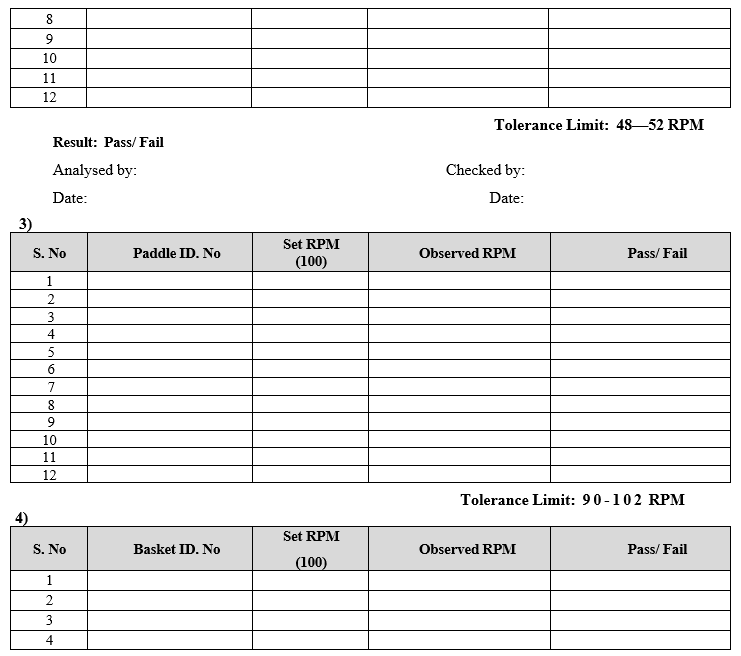
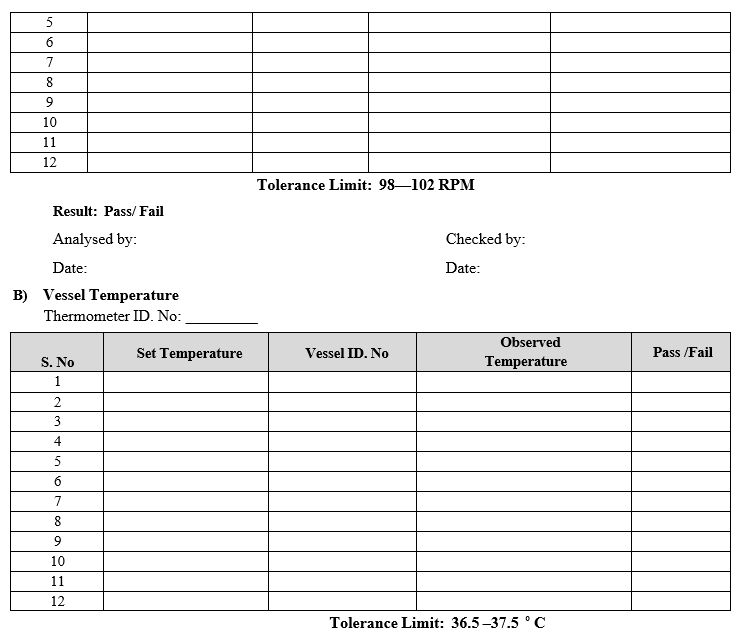
- Vessel Temperature Calibration:
- Keep the calibrated thermometer inside the water in all vessels and observe the temperature of the water.
- Actual temperature results shall be recorded in calibration format.
- Vessel Temperature Calibration:
Frequency: Quarterly
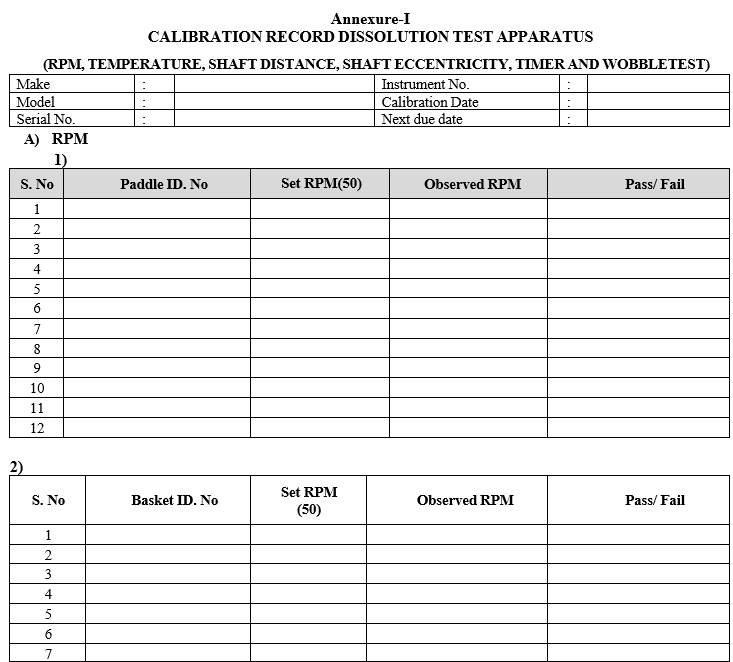
- Rotation Per Minute by Tachometer:
- Attach a small Magnetic piece to Paddle/Basket Shaft.
- Set the desired RPM value and start the Stirrer.
- Focus the light of Tachometer on Magnetic Piece.
- Allow the light from tachometer to fall sharply on Magnetic Piece and stand for one or two minutes.
- Observe the readings of RPM which are displayed on Tachometer. Repeat the Procedure for all paddle/basket shaft.
- Record the actual value in calibration format.
Frequency: Quarterly
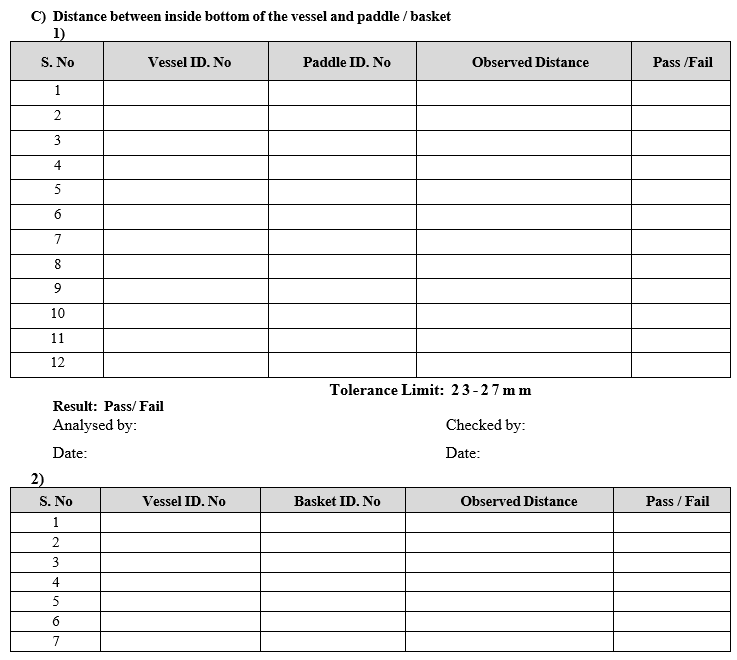
- Distance between the bottom edges of basket/paddle to the lowest inner surface of vessel.
- Empty all the vessels and keep them without lid in respective positions.
- Clamp all vessels using holders provided on jar plate.
- Lower the stirrer unit to the bottom most position till it stops automatically.
- Insert the depth gauge inside the vessel vertically.
- Hold the depth gauge vertically with both arms pressed such that the Vee bend takes the guide of the paddle shaft.
- Touch the reference jaw of the depth gauge at bottom face of paddle.
- Release the Arms and ensure that the lower side of the jaw touches the jar base.
- Note the reading of Vernier and main scale reading.
- Record the observation in calibration format.
Frequency: Quarterly
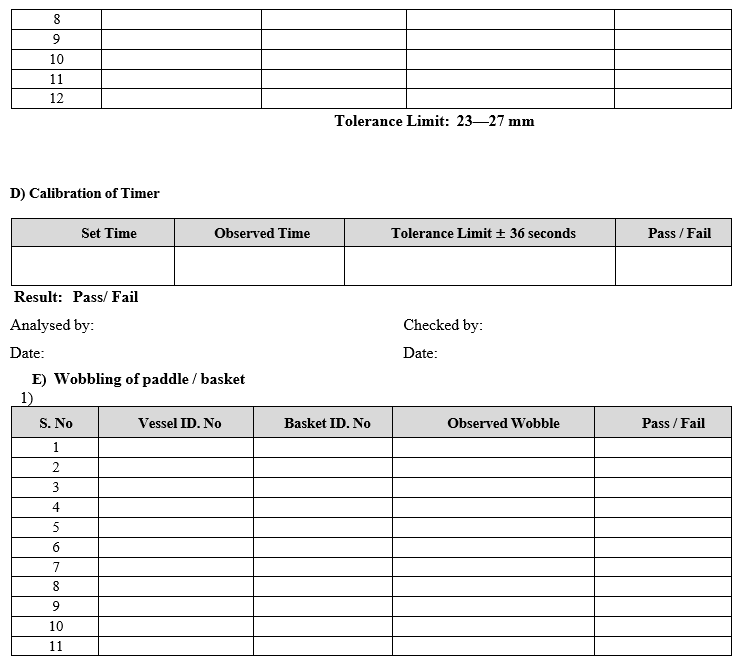
- Timer by stop clock:
- Set 30 minutes in instrument by pressing time key, start the instrument and Calibrated stop Clock. Simultaneously
- Record the actual value in calibration format.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-test-apparatus/
Frequency: Quarterly
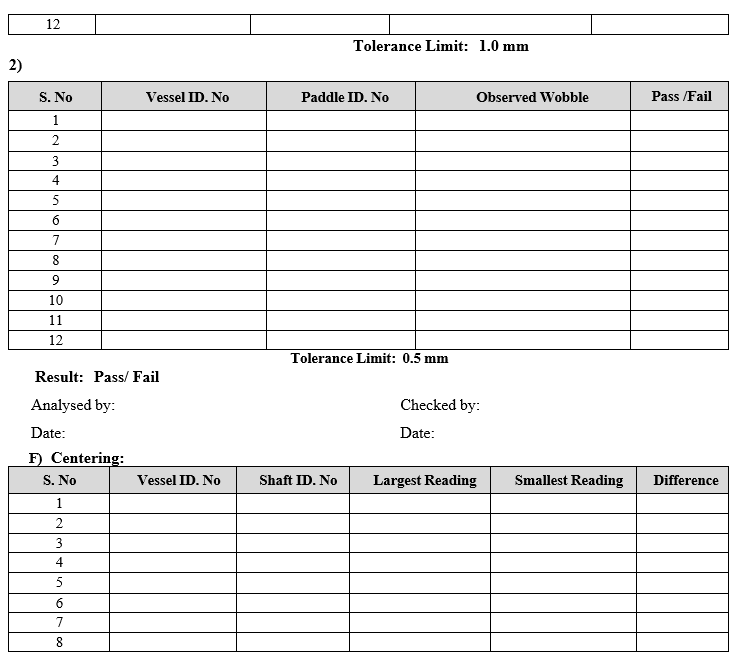
- Paddle/Basket Wobble Test:
- Ensure that the paddle or basket shaft is properly tightened with the spindle.
- Lift the stirrer up to a suitable level such that the paddle shaft/basket ring reaches up to measuring stylus point of dial gauge.
- For paddle: Fix the wobble meter on the vessel so that the pointer is just pressed against the shaft just above the paddle.
- For Basket: Fix the wobble meter on the vessel so that the pointer is just pressed against the bottom ring of the basket.
- Start the rotation of paddle/basket shaft.
- Check the maximum deviation of the pointer and note down the reading.
- Repeat the procedure for remaining paddles or basket shaft.
- Record the readings in calibration format.
Frequency: Quarterly
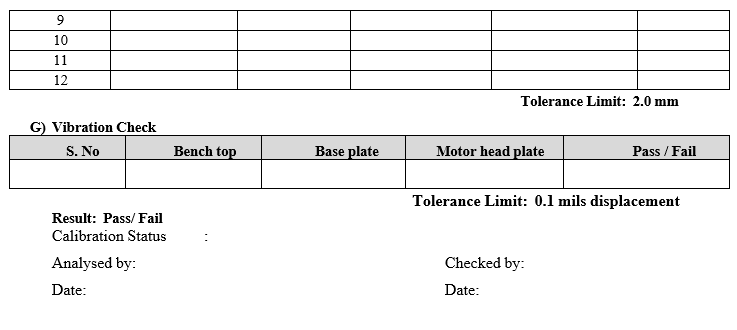
- Centering Test:Measure the distances from the shaft to the inner vessel wall at four locations equally spaced around the vessel and no more than 2 cm below the vessel flange. The difference between the largest and smallest readings is not greater than 2.0 mm. Repeat the above procedure for the remaining shafts. Record the readings in calibration format.
Frequency: Quarterly
- Vibration Check:
- Minimize vibration sources on the bench top to the extent possible.
- Switch off the motor and water bath circulation system before placing vibration meter.
- Before each test begins the surrounding area should be observed to detect and eliminate Obvious sources of vibration.
- Vibration should be checked on different places of tester such as Bench top, base plate and motor head plate.
- Record the observation in calibration format.
Frequency: Quarterly
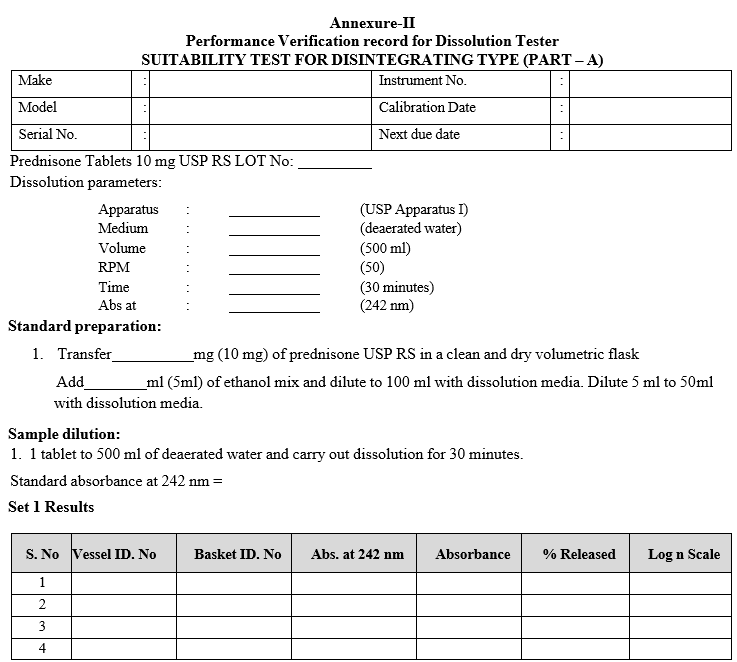
- Apparatus Suitability Test:
- It shall be carried out twice in a year or on change of dissolution vessels whichever is earlier.
- This is done by using current USP dissolution calibrator, USP Prednisone tablets.
- Method (This method is applicable for paddle as well as for basket)
| Medium | : | De-aerated water |
| Volume | : | 500 ml |
| Rotating speed | : | 50 rpm |
| Temperature | : | 37 ± 0.5°C |
| Time | : | 30 minutes |
- Transfer 10 mg of prednisone USP RS in a clean and dry volumetric flask and add 5ml of ethanol mix and dilute to 100 ml with dissolution media and further dilute 5 ml to 50 ml with dissolution media.
- Measure the absorbance of standard solution in a 1cm cell on suitable UV spectrophotometer at 242nm, using the dissolution medium as blank.
- Withdrawal sample from zone midway and filter sample through 0.45µm PVDF-type or equivalent filter and immediately measure the absorbance of sample solution.
- Calculate the amount of Prednisone dissolved in the dissolution medium by using the formula.
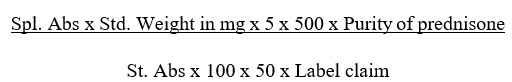
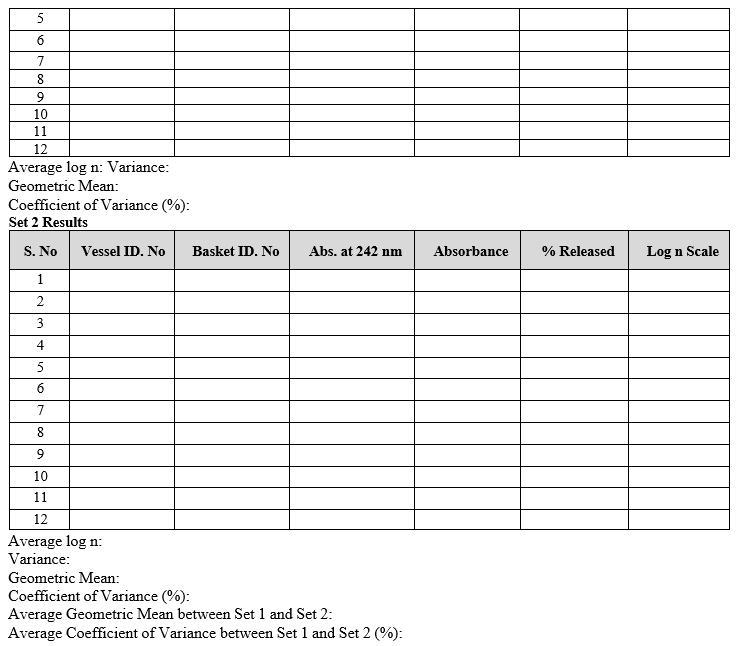
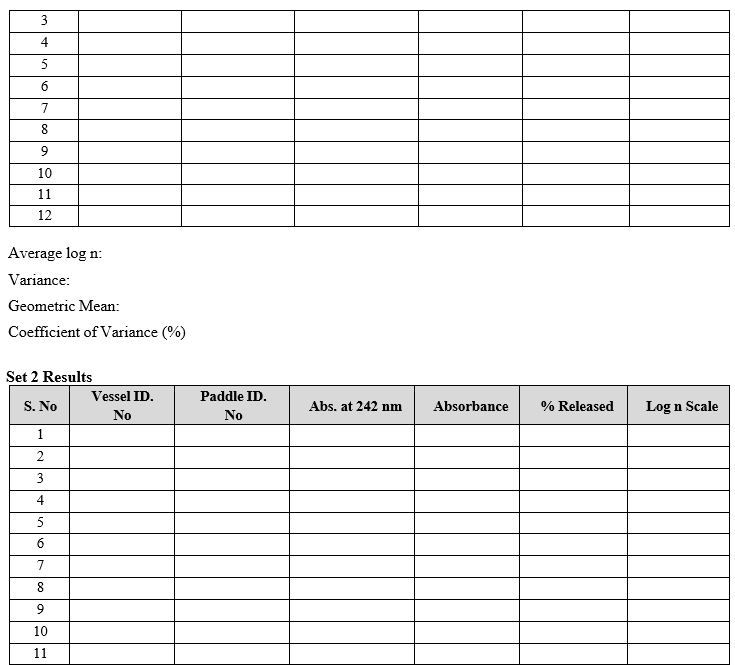
- Testing should be carried out on 12 units. For assemblies with lesser than 12 positions (Dissolution vessels), first conduct the testing on six units (set-1), and if the results satisfy the acceptance criteria, stop; the assembly has passed the apparatus suitability test. Otherwise continue the test with another 6 units (set-2). For assemblies with 12 positions no further testing is required.
- For assemblies with 12 positions: after calculating the % of Prednisone dissolved in dissolution media, convert the percentage results of Prednisone dissolved to log scale and determine mean and variance. Convert the results to a geometric mean (GM) and percent coefficient of variation (%CV) and round both to one decimal place.
- For assemblies lesser than 12 positions: after calculating the % of Prednisone dissolved, convert the results into a log scale and determine the mean and variance for set-1 and set-2 individually. Convert the results to a geometric mean (GM) and percent coefficient of variation (%CV) and round both to one decimal places.
- Record the calibration results in calibration format.
Frequency: Half yearly
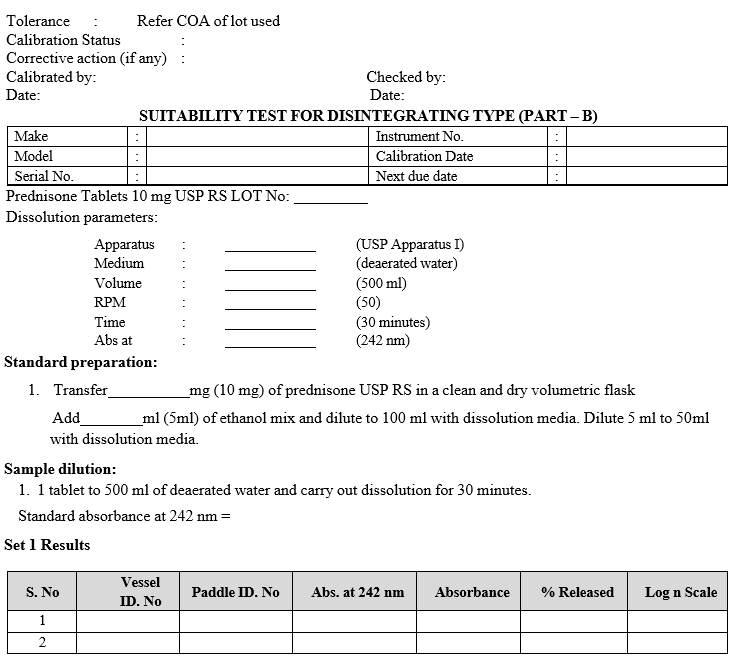
Acceptance Criteria: As per current USP Dissolution calibrator LOT.
- REFERENCES:
United States Pharmacopoeia
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Mechanical Calibration Record |
| Annexure-II | Performance Verification record for Dissolution Tester |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| USP | : | United States Pharmacopoeia |
| QC | : | Quality Control |
| RPM | : | Rotations Per Minute |
| CV | : | Coefficient of variation |
| GM | : | Geometric Mean |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-test-apparatus/
Annexure-I
CALIBRATION RECORD DISSOLUTION TEST APPARATUS
(RPM,TEMPERATURE, SHAFT DISTANCE, SHAFT ECCENTRICITY, TIMER AND WOBBLE TEST)
Annexure-II
Performance Verification record for Dissolution Tester
SUITABILITY TEST FOR DISINTEGRATING TYPE (PART – A)
Frequently Asked Question ?
Q: What are the key steps involved in setting up the instrument for dissolution testing?
A: Setting up the instrument involves ensuring the work area and apparatus are clean, operating the instrument per the SOP, filling the water bath and setting its temperature, installing the specified apparatus, filling dissolution jars with water, and allowing them to reach the specified temperature.
Q: What parameters of the dissolution test appratus need to be calibrated, and how often?
A: Several parameters require calibration, including:
- Physical parameters (quarterly):
- Jar temperature with thermometer
- Rotation speed with tachometer
- Distance between paddle/basket and vessel with depth gauge
- Paddle/basket wobble with wobble meter
- Centering with vernier caliper
- Timer with stop clock
- Vibration (quarterly): Minimize sources, check different areas with vibration meter.
- Apparatus Suitability Test (twice yearly or if vessels change): Use USP calibrator tablets and follow specific method to measure Prednisone dissolution and compare to acceptance criteria.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-test-apparatus/
