- OBJECTIVE:
- To lay down the procedure for adjustment of stock at the end of single lot number consignment.
- SCOPE:
- This SOP is applicable for the procedure for storage and handling of Expired and Rejected Finished Goods at {Company Name} {Location}.
- RESPONSIBILITY:
- WH Executive/Designee – shall be responsible to follow the procedure as per SOP.
- Head Warehouse – is responsible for compliance of the SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- During dispensing of Raw Materials and Packing Material, if the physical stock of current lot of material falls short/excess of the standard quantity (Labelled quantity); the same shall be updated as given below.
- Any handling loss/excess of Raw Material shall be within the acceptable limits as given under
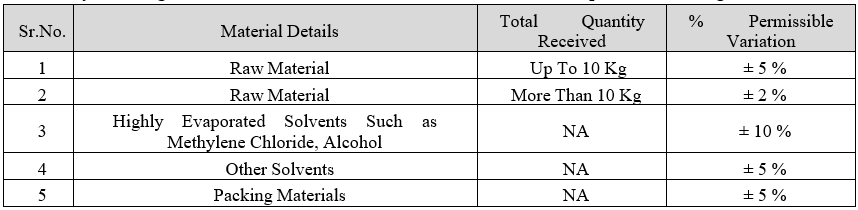
- Before dispensing the next lot of material, Executive/Designee Stores shall ensure that the shortage / excess found in the current lot is within the limit specified. If any material shortage/excess is not within the above given specified limit, then the detailed investigation shall be done and same shall be attached with the stock adjustment memo and in such cases take approval from the plant head before doing the entry.
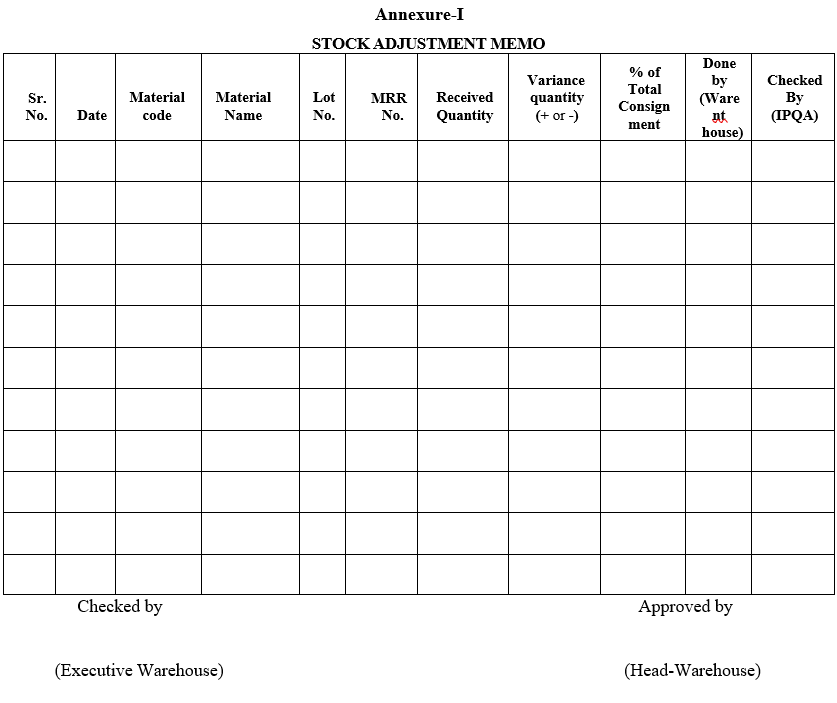
- If the shortage/excess is within the specified limit, Executive/Designee Warehouse shall record the details in Format-I ‘Stock Adjustment Memo’ before taking the next lot for dispensing.
- The stock adjustment will be done by Warehouse person, checked by the IPQA and the format is checked by Warehouse Executive and approved by the Warehouse Head before updating in system
- In case, the shortage/excess of stock exceed the above mentioned limit, and same will be updated with proper justification.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Stock Adjustment memo |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| MRR | : | Material Receipt report |
| QA | : | Quality Assurance |
| % | : | Percentage |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
STOCK ADJUSTMENT MEMO