- PROCEDURE:
- PREPARATION OF TEST SOLUTIONS:
- For all preparations of test solutions use purified water and Analytical Grade chemicals.
- Prepare the solutions as per the procedures given in general test procedures.
- Store the solutions in clean dry bottles at room temperature.
Note: Store light sensitive solutions in amber colour bottles
- A reference number shall represent all test solutions. The solution prepared frequently shall bear an AR number.
- Label the bottles as per the Format-I (Test solution label).
- All test solutions should be used within 3 months from the date of preparation.
- Fresh Test solution shall be prepared within 5 days on or before the ‘Valid Up to’ date.
- PROCEDURE FOR ISSUANCE AND IN-HOUSE NUMBERING OF THE HPLC COLUMN:
- A reference number shall represent each indicator solutions. Ex: For phenolphthalein indicator solution the reference number shall be QC/IS/01.
- where QC is Department code
- IS indicates indicator solution
- and 01 is the reference number.
- The solution prepared frequently shall bear an AR number. Eg: Phenolphthalein indicator solution initially shall have a number QC/IS/01/2501
- where
- QC is department code
- IS is indicator solution
- 01 reference number
- ‘25’ represents the year
- and ‘01’ represents the serial number which shall be a continuous number i.e.., 02, 03,…and so on.
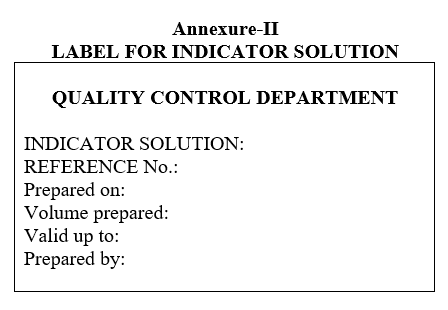
- Label the indicator solutions as per the Format-II (Label for indicator solution).
- All indicator solutions should be used within 6 months from the date of preparation.
- Ready to use indicators does not require any preparation. They shall be used directly after checking the sensitivity given in general test procedures. These solutions should be used within 12 months from the date opened.
- PROCEDURE FOR LIMIT TEST STANDARD SOLUTIONS:
- Follow the procedure described in test solutions.
- Proportionately smaller or larger quantities of chemicals shall be weighed accurately, provided the measurement is made with at least equivalent accuracy and provide any subsequent steps, such as dilutions are adjusted to yield concentrations, equivalent to those intended solutions.
- A reference number shall represent each Limit Test solution. Ex: For Lead standard solution the reference number shall be QC/LS/01
- where
- QC is Department code
- LS is limit test standard
- and 01 is the reference number.
- The solution prepared frequently shall bear an AR number. Ex: Lead standard solution initially shall have a number QC/LS/01/2201
Where
- QC is Department code
- LS is limit test standard
- 01 is the reference number
- ‘22’ represents the year
- and ‘01’ represents the serial number which shall be a continuous number i.e., 02, 03…. And so on.
- Label the solution as per the Format-III (Label for limit test standard solution)
- Specify the Concentration of the solution on the label.
- All limit test standard solutions should be used within three months from the date of preparation.
- Fresh Limit Test solutions shall be prepared within 5 days on or before the ‘Valid Up to’ date.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Label for test solution |
| Annexure-II | Label for indicator solution |
| Annexure-III | Label for Limit Test solution |
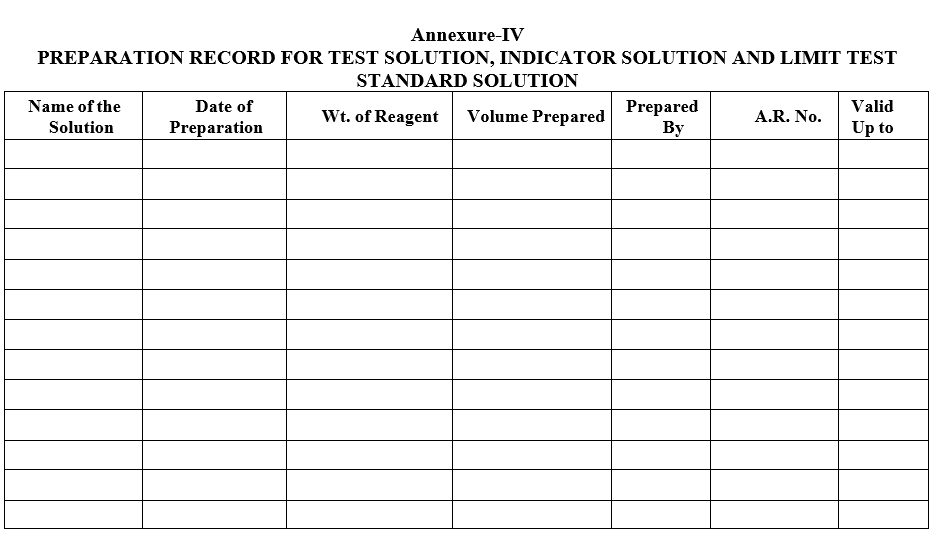
| Annexure-IV | Preparation record for Test solution, Indicator Solution and Limit Test Standard solution |
Annexure-I
LABEL FOR TEST SOLUTION

Annexure-II
LABEL FOR INDICATOR SOLUTION
Annexure-III
LABEL FOR LIMIT TEST SOLUTION

Annexure-IV
PREPARATION RECORD FOR TEST SOLUTION, INDICATOR SOLUTION AND LIMIT TEST STANDARD SOLUTION