- PROCEDURE:
- Layouts shall be prepared for the following but not limited to
- PROCEDURE:
- Layouts shall be prepared for the following but not limited to
- Facility Layout
- Equipment Layout
- Utilities layout (like HVAC, Water System, Compressed Air System)
- Facility Layout includes the following but not limited to
- Site layout
- Different Area/section/ modules
- Man Movement layout.
- Material Movement layout
- Drain Point Location layout.
- Pest and Rodent control layout
- Equipment layout includes the following but not limited to
- Location of equipment in area.
- Schematic arrangement of the equipment
- Utility layout includes the following but not limited to
- Schematic arrangements of AHU, dust collector
- Duct layout, grill layout.
- Pressure Gradient layout, AHU Zoning layout, Area classification layout, P&ID of Water or Compressed Air Systems, AHU Schematic drawing.
- Piping and Instrumentation diagram
- Preparation of layout
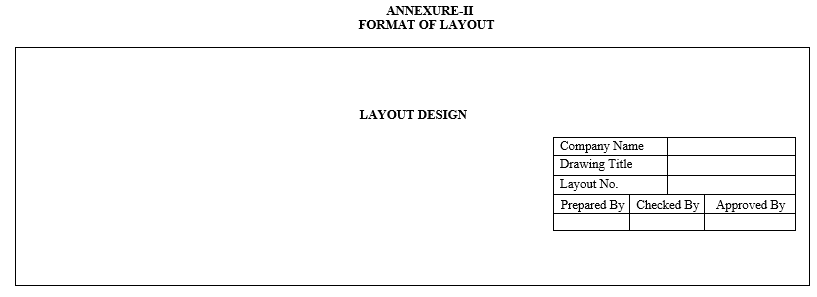
- Engineering / Project department shall prepare the draft copy of layout A3 or A4 as per the format detailed in Annexure-II in consultation with user department layouts depicting only the outline of facility, rooms shall indicate the scale and the units.
- Any short forms, diagrammatic representations, colour codes shall be explained in the layouts.
- Engineering /project department shall put ‘DRAFT COPY’ stamp and shall circulate to the technical/user departments and QA for review.
- Upon receipt of draft copy, the reviewer shall review and send comments to the Engineering/Project department for incorporating the same in the final layout.
- After incorporation of suggestion(s) as applicable, print of layout (QA copy, Display copies as applicable & Site Master File copy as applicable) shall be taken and shall be forwarded for signatures from Technical/User department and final approval from Plant QA In-charge or designee.
- Engineering / Project department shall destroy the draft copy by shredding after the approval of the layout as per Annexure-II
- In case of additional original copy, reprint shall be taken and shall be reviewed by Technical/User department and approved by Plant QA In-charge.
- The layouts prepared by external consultants shall be verified and shall be numbered as detailed in section
- Engineering / Project department shall do the verification and approval.
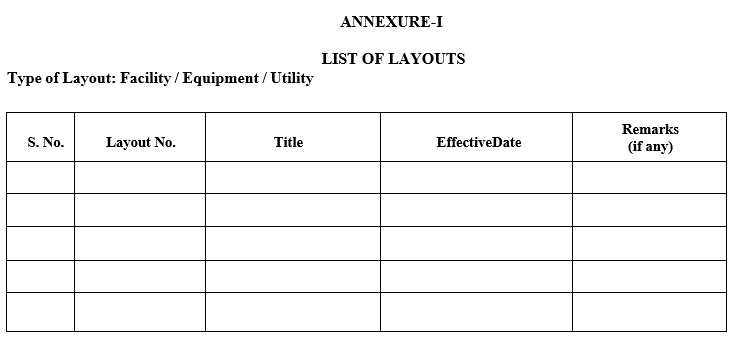
- All approved layouts shall be maintained as per Annexure-I. (List of layouts).
- Issuance of layouts
- Approved layout copies shall be submitted to QA for issuance of the same.
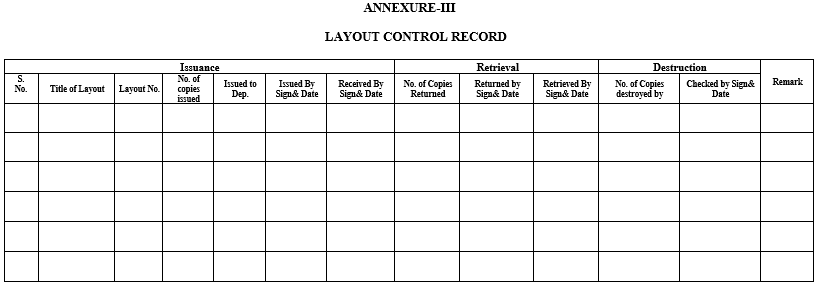
- Documentation person shall enter the layout number and name in the logbook as detailed in Annexure-III.
- User department shall raise document Requisition/Distribution slip, get it approved by Plant QA In-charge or designee.
- QA shall issue the Photocopy of the layout as a DISPLAY copy and shall take the Signature of the receiver in the logbook.
- In case, the layout is required to be issued to external agencies, regulatory bodies, customers, additional original copy, reprint shall be taken and shall be reviewed by Technical/User department and approved by Plant QA In-charge or Photocopy of QA copy or SMF copy shall be taken and issued with stamp Reference copy – Not for operational use.
- Revision of Layouts
- The existing layout, if revised, due to any changes/modification in the facility or system or equipment location, then Initiator department shall raise the change control with necessary justification.
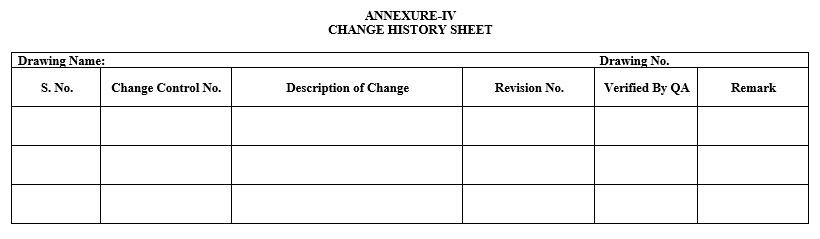
- After approval of the Change Control, Layout shall be revised, and the changes shall be recorded in the change history as per Annexure-IV.
- All the existing controlled copies of layout shall be retrieved and forwarded to QA by concerned department(s).
- Upon receipt of the retrieved copy, QA shall record and destroy the retrieved copy (ies) by shredding and maintain the record for the same.
- QA copy of the layout shall be maintained by putting the stamp “OBSOLETE” in red on the layout and filed separately.
- Plant QA shall control, issue, and retrieve the layouts and maintain records.
- Numbering of layouts
- A unique number shall be assigned for layouts.
- AAA/BBB/C/NNN-XX
- AAA: Indicates Company name abbreviations.
- BBB: Indicate Layout
- C: Indicates for if facility layout the Facility Layout denotes as “F” , `E` for Equipment layout `U` for Utility layout
- NNN: Indicates serial no of layout start from 001,002, 003……
- XX: Indicates version number of layout starting from 00 and continuing serially in increments of one unit.
- For e.g. the facility layout no shall be given as AAA/LYT/F/001-00
- There shall be separate series for each type of layout i.e. Facility, Equipment and Utility.
- REFERENCES:
- Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | List of Layouts |
| Annexure-II | Format of Layout |
| Annexure-III | Layout Control Record |
| Annexure-IV | Change History Sheet |
- ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 Head Quality Assurance
- Controlled Copy No. 02 Head-Engineering
- Master Copy Quality Assurance Department
- ABBREVIATIONS:
- P&I : Piping and Instrumentation
- HVAC : Heating Ventilation and Air-conditioning
- AHU : Air Handling Unit
- QA : Quality Assurance
- REVISION HISTORY:
- CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Annexure-I
LIST OF LAYOUTS
Annexure-II
FORMAT OF LAYOUT
Annexure-III
LAYOUT CONTROL RECORD
Annexure-IV
CHANGE HISTORY SHEET