
OBJECTIVE:
To lay down a procedure for preparation of site master file, Manuals and master plans.
SCOPE:
This SOP is applicable for Preparation, Review, Approval, Control, Issuance, Retrieval, Revision and Destruction of Obsolete of Site Master File, Training Manual, Quality Manual, Validation Master Plan, Cleaning Validation Master Plan, Safety Manual & On-Site Emergency Plan at {COMPANY NAME} {COMPANY LOCATION}.
RESPONSIBILITY:
Operating Officer /Executive shall be responsible for preparation of Site Master File, Manuals and Master Plans.
Operating Manager / Designee shall be responsible for review of Site Master File, Manuals and Master Plans.
QA Officer/ Executive shall be responsible for Controls the Issuance, Revision, Retrieval and Destruction of Obsolete of Site Master File, Manuals and Master Plans.
ACCOUNTABILITY:
Head QA shall be accountable for Approval, Implementation and Control in all the Applicable Areas.
PROCEDURE:
GENERAL GUIDELINES FOR PREPARATION OF SITE MASTER FILE, TRAINING MANUAL, QUALITY MANUAL, VALIDATION MASTER PLAN, CLEANING VALIDATION MASTER PLAN, SAFETY MANUAL & ON-SITE EMERGENCY PLAN. PREPARATION OF ABOVE MENTIONED DOCUMENTS, INITIATORS SHALL FOLLOW FOLLOWING GUIDELINES / INSTRUCTIONS:
The all documents shall be written in English Language by using Microsoft Word typing.
All Documents shall be initiated by QA Department.
Documents shall be written after thorough understanding of the Subject. The Initiators of the Documents shall have adequate Knowledge, Training and Experience in the related Areas of Operations.
Initiator shall check the completeness of draft Documents and send the hardcopy to the concerned Department Head / Operating Manager (who would be directly affected by the Documents) for review.
The reviewer shall review the draft Documents for Accuracy of the Subject Matter & Logical Sequence of Instructions.
On receipt of the comments (if any), the same shall be reviewed and incorporated in the documents if agreed.
The draft Documents after completion of reviewing and incorporation of comments or suggestions as agreed, shall be destroyed, this shall be done in concerned Originating Department.
Master Copies of all documents shall be printed in QA Department only.
Documents should be handled with care and should not be Spoiled or Torn.
No Manual Correction shall be made in Approved Copy (Master / Controlled Copy).
Storage of Master Copy / Soft Copy: All Master Copy / Soft Copy of Approved Documents shall be stored in QA Department with Password Protected System and Data Backup shall be kept in Information Technology (IT) Department.
Wherever necessary Illustrations and Drawing shall be indicated to provide better clarity and understanding of the Process / System
Font size of table Content may be Changeable in case of insufficient space but it should be not less than 9 Font.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-of-site-master-file-manuals-and-master-plans/
All Manuals, Master Plan and SMF contents shall be covered by Double Borderline with ½ Line Weight (Width).
Line Spacing and Font Size: The Line Spacing between two points or Title & Subtitle shall be minimum Single Line. Font Style shall be Times New Roman 12 font. Font size may be less.
All Manuals, Master Plan and SMF shall be printed on A4 size White Color Glossy Paper of 100 GSM (Except Cover Page, Cover Page shall be printed on A4 Size White Photo Paper of 180 GSM) with Color Printing using “Times New Roman” Font. Printing shall be done on one side of the paper only.
Paper Selection for Print: Paper 8.5” (Width), 11.5” (Height), Border Top 24pt, Left 24pt, Right 24pt and Bottom 31pt with A4 Size Scaling.
All Manuals, Master Plan and SMF shall be binded in Spiral Binding with A4 size transparent OHP sheet on both sides as outer cover.
Content of Header and Footer:
Header:
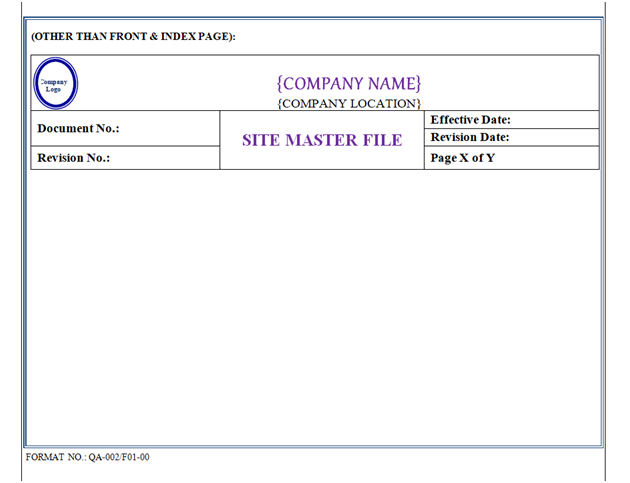
The Header of Site Master File shall have the Name of Organization (Including Name of Location). Header shall have the “Logo” of Organization in Left corner on Top, and followed by “Site Master File” in centerwritten in Bold and Capital letter of font size 16 as shown in Annexure-I.
Document No.: Document No.Shall be written in Bold and Running Letter of Font Size 12.
Revision No.: Revision No.Shall be written in Bold and Running Letter of Font Size 12.
Effective Date: Effective Date shall be assigned in the form of DD/MM/YY & shall be written in bold and running Letter of Font Size 12.
Revision Date: Revision Date shall be assigned in the form of DD/MM/YY & shall be written in bold and running Letter of Font Size 12.
Page No.: Shall be written in Normal of Font Size 12. The Page Number shall be mentioned in ‘X of Y’ format.
For Example: If a Site Master File contains 20 pages then the first page of the Site Master File shall be 1 of 20 and the second page shall be 2 of 20 respectively & shall not be printed on Front & Index Pages.
Footer:
Format No.: 09 Normal & Capital font size printed on the Left Corner of the page after Footer, out of page border and shall be printed.
Example: For Site Master File format no. shall be QA-002/F01-00 on all pages.
FONT STYLE / SIZE OF HEADER, FOOTER & BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| Header: | |
| Name of the Organization | 20 Bold & Capital in Red Colour |
| Location ({COMPANY LOCATION}) | 12 Capital & Normal in Black Colour |
| Logo with Company Name (On Left Hand Side Corner of The Page) | Logo– Height-0.75’’& Width-0.65’’ |
| Documents Name | 16 Capital & Bold in Blue Colour |
| Document No. | 12 Normal & Bold in Black Colour |
| Revision No. | 12 Normal & Bold in Black Colour |
| Effective Date | 12 Normal & Bold in Black Colour |
| Revision Date | 12 Normal & Bold in Black Colour |
| Page No. | 12 Normal & Bold in Black Colour |
| Body other than Front Page | |
| Heading | 12 Capital / Normal & Bold in Blue / Black Colour |
| Content other than Heading | 12 Normal & in Black Colour |
| Footer: | |
| Format No. | 09 Capital & Normal in Black Colour |
* Font size of body other than front page can be change as per requirement but it should be not less than 12 font.
* For SMF Header, Footer & Body shall be written in point no.5.2.4.
APPROVAL:
This Quality Document “DOCUMENT NAME” elaborates the organization, and describes the Manufacturing Facility & Quality Management System being followed in {COMPANY NAME}, {COMPANY LOCATION}.
PREPARED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| ASST. MANAGER/DESIGNEE (OPERATING) | – | – | – |
REVIEWED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| HEAD (OPERATING) | – | – | – |
APPROVED BY:
| DESIGNATION | NAME | SIGNATURE | DATE |
| HEAD (QUALITY ASSURANCE) | – | – | – |
Note: In SMF, Manuals & Master plans, the heading & sub headings, mentioned in standard format can be deleted or new heading & sub heading can be added as per requirement.
SITE MASTER FILE:
Site Master File shall contain adequate information. Simple plans outline drawings or schematic layouts are preferred instead of narratives. Site Master File, including Appendix, shall be readable when printed on A4 paper sheets.
Layout of Site Master File (SMF):
Format Considerations:
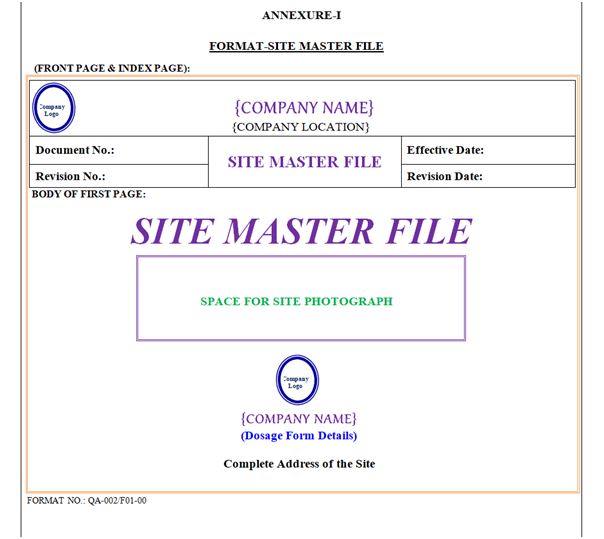
SMF shall contain Header, Footer and Body. “Site Master File Format” shown in Annexure-I.
All the points in the SMF shall be numbered sequentially and sub paragraph of the SMF be also numbered sequentially with an incremental number derived from the Heading Number.
SMF Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of SMF shown in Annexure-I.
Content of the Body:
Front Page: First page body shall have word “Site Master File” font style shall be Times New Roman 36 font (Normal Italic Bold). Photo of Manufacturing Site & Location Name (16 Font) & Address (12 Font) with Logo shown in Annexure-I.
Index of Site Master File: Indexin the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
| Section | Description | Page No. |
| – | – | – |
CONTENT OF THE SITE MASTER FILE:
APPROVAL:
GENERAL INFORMATION OF THE MANUFACTURER: Brief description of name, address, contact information of the manufacture, import, export, distribution etc.
QUALITY MANAGEMENT SYSTEM OF THE MANUFACTURER: Brief description of the Quality Management Systems, Qualification Requirements (Education and Work Experience) of the Authorized Person(s) / Qualified Person(s), Management of Suppliers and Contractors, Quality Risk Management (QRM)Methodologies & Product Quality Reviews
PERSONNEL: Description about Organization
PREMISES AND EQUIPMENT: Short description of Plant Premises, Heating, Ventilation and Air Conditioning (HVAC) Systems, Water Systems, Equipment, Utilities Cleaning and Sanitization & GMP Critical Computerized Systems
DOCUMENTATION: Description of Documentation System (i.e. Electronic, Manual).
PRODUCTION: Brief description of production.
QUALITY CONTROL (QC): Description of the Quality Control activities carried out on the site in terms of Physical, Chemical, and Microbiological Testing.
DISTRIBUTION, COMPLAINTS, PRODUCT DEFECTS AND RECALLS: Brief Description of the System for Handling Complains Product defects and Recalls
SELF INSPECTIONS: Short description of the Self Inspection.
REFERENCES: Provide the details of the references which are used for the preparation of the SMF.
APPENDIX: Attachthe entire Appendix (Not limited to may be change as per Plant Requirement).
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-of-site-master-file-manuals-and-master-plans/
FONT STYLE / SIZE OF HEADER, FOOTER & BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| Header of First & Index Page: | |
| Name of the Organization | 20 Bold & Capital (Hobo BT) in Red Colour |
| Location ({COMPANY LOCATION}) | 12 Capital & Normal in Black Colour |
| Logo with Company Name (On Left Hand Side Corner of The Page) | Logo– Height-0.75’’& Width-0.65’’ |
| Site Master File | 16 Capital & Bold in Blue Colour |
| Document No. | 12 Normal & Bold in Black Colour |
| Revision No. | 12 Normal & Bold in Black Colour |
| Effective Date | 12 Normal & Bold in Black Colour |
| Revision Date | 12 Normal & Bold in Black Colour |
| Page No. | 12 Normal & Bold in Black Colour |
| Body Front Page: | |
| Site Master File | 36 Normal, Bold & Italic in Blue Colour |
| Location Name | 16 Capital & Bold (Hobo BT) in Red Colour |
| Dosage Form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
| Header Other than Front & Index Page: | |
| Name of the Organization | 20 Bold & Capital (Hobo BT) in Red Colour |
| Location ({COMPANY LOCATION}) | 12 Capital & Normal in Black Colour |
| Logo with Company Name (On Left Hand Side Corner of The Page) | Logo– Height-0.75’’& Width-0.65’’ |
| Site Master File | 16 Capital & Bold in Blue Colour |
| Document No. | 12 Normal & Bold in Black Colour |
| Revision No. | 12 Normal & Bold in Black Colour |
| Effective Date | 12 Normal & Bold in Black Colour |
| Revision Date | 12 Normal & Bold in Black Colour |
| Page No. | 12 Normal & Bold in Black Colour |
| Footer: | |
| Format No. | 09 Capital & Normal in Black Colour |
Numbering System of Site Master File:
Site Master File shall contain Seven Alphanumeric Characters (Three Alphabets, Three Numeric and One Separators). Site Master File shall be assigned with a Unique Number, once number is allotted to Site Master File, the same number shall not be assigned.
For Example: First Site Master File shall be numbered as SMF/001
Where:
| SMF | : | Indicate the code of Site Master File |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Site Master File |
TRAINING MANUAL:
Layout of Training Manual:
Format Considerations:
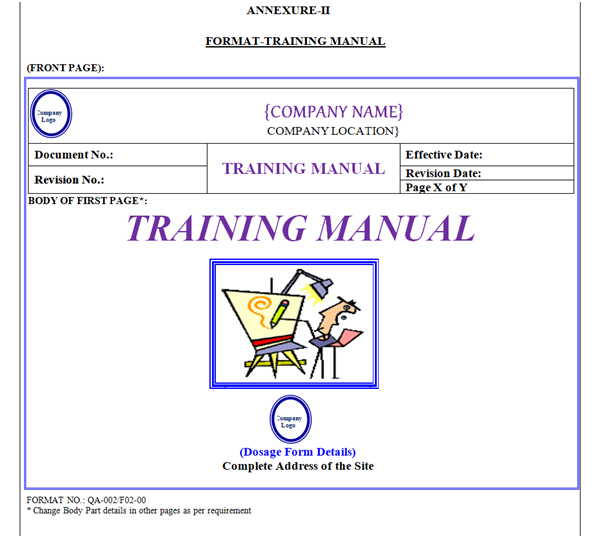
Training Manual shall contain Header, Footer and Body. “Training Manual” shown in Annexure-II.
All the points in the Training Manual shall be numbered sequentially and sub paragraph of the Training Manual be also numbered sequentially with an incremental number derived from the heading number.
Training Manual Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of Training Manual shown in Annexure-II.
Content of the Body:
Front Page: First page body shall have word “Training Manual” font style shall be Times New Roman Italic 36 font. Photograph of Training Symbol, Location Name & Address with Logo as shown in Annexure-II.
Table of Contents: Training Manual shall contain “Table of Contents” in the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
| Section | Description | Page No. |
| – | – | – |
Contents of Training Manual:
Approval:
Introduction: Brief about the Company including Location & Certification details, Mission, Vision, Core Values, Quality Policy, Health Safety & Environment Policy & Objectives
Training Policy: Brief Description of Training & Development, Aims & Objectives, Training Committee/ Team, Levels of Trainee, Identification of Trainer, Training Module, Training Plan, Types of Training & Evaluation of Trainees
Process Flow Diagrams.
Basics of cGMP and Introduction to Schedule-M. Brief description of Fundamentals of GMP, Why GMP, Requirements of GMP, Ten Golden Rules of GMP, Description of Schedule-M, Building Facilities, Equipment, Water System & HVAC System, Sanitization & Personnel Hygiene, Standard Operating Procedure (SOP), BMR/ BPR, Documentation, Validation, Packing & Labeling, Maintenance Programme, Basics of GLP & Role of Quality Control Department, Self Inspection, Complaints, Recall & Withdrawals
Glossary: Provide the details of Glossary.
Revision History: Shall provide the revision details of Training Manual & shall contain the following details:
| S. No. | Effective Date | Revision No. | Reason for Revision |
| – | – | – | – |
FONT STYLE / SIZE OF BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| Body First Page: | |
| Training Manual | 36 Normal & Bold (Italic) In Red Colour |
| Location Name | 16 Capital & Bold In Red Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of Training Manual: Training Manual shall contain Six Alphanumeric Characters (Two Alphabets, Three Numeric and One Separators). Training Manual shall be assigned with a Unique Number, once number is allotted to Training Manual, the same number shall not be assigned.
For Example: First Training Manual shall be numbered as TM/001
| TM | : | Indicate the code of Training Manual |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Training Manual |
QUALITY MANUAL:
Layout of Quality Manual:
Format Considerations:
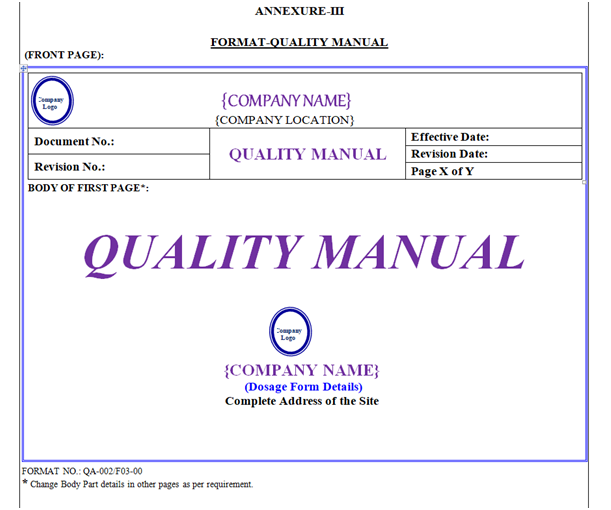
Quality Manual shall contain Header, Footer and Body. “Quality Manual” shown in Annexure-III.
All the points in the Quality Manual shall be numbered sequentially and sub paragraph of the Quality Manual be also numbered sequentially with an incremental number derived from the heading number.
Quality Manual Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of Quality Manual shown in Annexure-III.
Content of the Body:
Front Page: First page body shall have word “Quality Manual” font style shall be Times New Roman Italic 36 font. Symbol of Quality, Location Name & Address with Logo as shown in Annexure-III.
Index of Quality Manual: First Page of Quality Manual shall contain “Table of Contents” in the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
| Section | Description | Page No. |
Contents of Quality Manual:
- Approval :
- Introduction:
- Quality Management System: Brief description ofQuality Policy, Product life cycle, Sis System Approach & Production System
- General Information: Individual Brief Personnel Profiles of QA & QC Personnel.
- Responsibility of Quality Assurance Department:
- Responsibilities of Quality Control Department:
- Documentation Management:
- Management Responsibilities:
- Resource Management
- Production Realization:
- Measurement , Analysis and Improvement:
- Water System:
- Reference:
- Appendix:
- Revision History:
Shall provide the revision details of Quality Manual & shall contain the following details:
| S. No. | Effective Date | Revision No. | Reason for Revision |
| – | – | – | – |
FONT STYLE / SIZE OF BODY CONTENTS:
| NAME OF CONTENT | FONT SIZE |
| BODY FIRST PAGE: | |
| Quality Manual | 36 Normal & Bold (Italic) In Red Colour |
| Location Name | 16 Capital & Bold In Red Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of Quality Manual:
Quality Manual shall contain Six Alphanumeric Characters (Two Alphabets, Three Numeric and One Separators). Quality Manual shall be assigned with a Unique Number, once number is allotted to Quality Manual, the same number shall not be assigned.
For Example: First Quality Manual shall be numbered as QM/001.
Where,
| QM | : | Indicate the code of Quality Manual |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Quality Manual |
VALIDATION MASTER PLAN:
Layout of Validation Master Plan (VMP):
Format Considerations:
VMP shall contain Header, Footer and Body. “Validation Master Plan” shown in Annexure-IV.
All the points in the VMP shall be numbered sequentially and sub paragraph of the VMP be also numbered sequentially with an incremental number derived from the heading number.
Validation Master Plan Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of VMP shown in Annexure-IV.
Content of the Body:
Front Page: First page body shall have word “Validation Master Plan” font style shall be Times New Roman 55 font. Location Name & Address with Logo.
First Page Body Part details shown in Annexure-IV.
Index: Validation Master Plan shall contain “Table of Contents” in the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
| Section | Description | Page No. |
| – | – | – |
Contents of Validation Master Plan:
- Approval:
- General Information: Brief Description of Facility & Manufacturing Activities & General Description Building.
- Validation Master Plan (VMP): Brief description of Objective of the VMP, Requirement of Validation, Over view of VMP, Activity Matrix, Equipment Qualification Process & Process Validation Flow.
- Validation Documentation:
- Area Qualification Approach: Brief description of Qualification & Minimum Requirement of Area Qualification (AQ) & Protocol.
- Qualification Approach: Brief description of Design Qualification of Area, Installation Qualification of Area, Operational Qualification (OQ) of Area & Performance Qualification (PQ) of Area.
- Equipment Qualification Approach: Brief description of equipment qualification.
- Qualification Approach: Brief description of URS, FAT, SAT, IQ, OQ & PQ of Equipment / Utilities / Facilities.
- Performance Qualification (PQ) of Utilities: Brief description of HVAC, Water System, Compressed Air, Process Validation Approach, Hold Time Study Approach,
- Cleaning Validation:
- Computer System Validation:
- Change Control / Planned Modification:
- Document Management:
- Validation & Qualification Frequency:
- Revision History:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-of-site-master-file-manuals-and-master-plans/
| S. No. | Effective Date | Revision No. | Reason for Revision |
| – | – | – | – |
FONT STYLE / SIZE OF BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| BODY FIRST PAGE: | |
| Validation Master Plan | 55 Normal & Bold (Italic) in Red Colour |
| Location Name | 16 Capital & Bold In Black Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of Validation Master Plan: Validation Master Plan shall contain Seven Alphanumeric Characters (Three Alphabets, Three Numeric and One Separators). Validation Master Plan shall be assigned with a Unique Number, once number is allotted to Validation Master Plan, the same number shall not be assigned.
For Example: First Validation Master Plan shall be numbered as VMP/001
Where,
| VMP | : | Indicate the code of Validation Master Plan |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Validation Master Plan |
CLEANING VALIDATION MASTER PLAN:
Layout of Cleaning Validation Master Plan:
Format Considerations:
Cleaning Validation Master Plan shall contain Header, Footer and Body. “Cleaning Validation Master Plan” shown in Annexure-V.
All the points in the Cleaning Validation Master Plan shall be numbered sequentially and sub paragraph of the Cleaning Validation Master Plan be also numbered sequentially with an incremental number derived from the Heading Number.
Cleaning Validation Master Plan Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of Cleaning Validation Master Plan shown in Annexure-V.
Content of the Body:
Front Page: First page body shall have word “Cleaning Validation Master Plan” font style shall be Times New Roman Italic 55 font. Location Name & Address with Logo shown in Annexure-V.
Index of Cleaning Validation Master Plan: Cleaning Validation Master Plan shall contain “Table of Contents” in the form of given table. Header Row of Table shall have gray 25 % shading Color.
| Section | Description | Page No. |
| – | – | – |
Contents of Cleaning Validation Master Plan:
- Approval:
- Introduction:
- Objective:
- Scope:
- Cleaning Validation Team & Responsibility: Flow Charts of the Cleaning Validation Development for a Particular Product / Equipment.
- Product / Equipment Grouping:
- Matrixing:
- Cleaning Type:
- Selection of Analytical Method:
- Selection of Cleaning Method: (i) Clean-in-Place (CIP) Method. (ii) Clean-Out-of-Place (COP) Method. (iii) Manual Cleaning Method.
- Selection of Sampling Method: (i) Rinse Sampling. (ii) Swab Sampling.
- Cleaning Agents:
- Sampling Plan:
- Establishment of Limit and Acceptance Criteria for Maximum allowable Carry-Over for Previous Product Residue:
- Establishing Maximum Allowable time Limit for Storage of Cleaned Equipment Before use:
- Cleaning Validation Protocol:
- Cleaning Validation Report:
- Revalidation Criteria:
- Cleaning Validation Frequency:
- Glossary:
- Revision History: Shall provide the revision details of Cleaning Validation Master Plan & shall contain the following details:
| S. No. | Effective Date | Revision No. | Reason for Revision |
| – | – | – | – |
FONT STYLE / SIZE OF BODY CONTENTS :
| NAME OF CONTENT | FONT SIZE |
| Body First Page: | |
| Cleaning Validation Master Plan | 36 Normal & Bold (Italic) in Red Colour |
| Location Name | 16 Capital & Bold In Black Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of Cleaning Validation Master Plan:
Cleaning Validation Master Plan shall contain Eight Alphanumeric Characters (Four Alphabets, Three Numeric and One Separators). Cleaning Validation Master Plan shall be assigned with a Unique Number, once number is allotted to Cleaning Validation Master Plan, the same number shall not be assigned.
For Example: First Cleaning Validation Master Plan shall be numbered as CVMP/001
Where,
| CVMP | : | Indicate the code of Cleaning Validation Master Plan |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Cleaning Validation Master Plan |
SAFETY MANUAL:
Layout of Safety Manual:
Format Considerations:
Safety Manual shall contain Header, Footer and Body. “Safety Manual” shown in Annexure-VI.
All the points in the Safety Manual shall be numbered sequentially and sub paragraph of the Safety Manual be also numbered sequentially with an incremental number derived from the heading number.
Safety Manual Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of Safety Manual shown in Annexure-VI.
Content of the Body:
Front Page: First page body shall have word “Safety Manual” font style shall be Times New Roman 65 font in Italic with symbol of Environment Health & Safety as shown in Annexure-VI. Location Name & Address with Logo. First Page Body Part details shown in Annexure-VI.
Index of Safety Manual: First Page of Safety Manual shall contain “Table of Content” in the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
| S. No. | Contents | Page No. |
| – | – | – |
Contents of Safety Manual:
- Approval:
- Introduction:
- Environment Health & Safety Policy:
- Objectives:
- Responsibility:
- Safety Principles:
- Basic Safety Rules:
- General Safety:
- Our Aim Zero Accidents:
- Accident Intimation and Post Accident Measures:
- Disasters:
- Emergency Exits from Plant:
- Fire Extinguishers:
- Safety Trainings:
- Accidents & Emergencies:
- Environment Pollution & Control:
- Reference:
- Glossary:
- Appendix:
| S. No. | Title | Appendix No. |
| – | – | – |
20. Revision History:
| S. No. | Effective Date | REVISION No. | Reason for Revision |
| – | – | – | – |
Font Style / Size of Body Contents :
| NAME OF CONTENT | FONT SIZE |
| BODY FIRST PAGE: | |
| Safety Manual | 65 Normal & Bold (Italic) in Red Colour |
| Location Name | 16 Capital & Bold In Red Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of Safety Manual:
Safety Manual shall contain Six Alphanumeric Characters (Two Alphabets, Three Numeric and One Separators). Safety Manual shall be assigned with a Unique Number, once number is allotted to Safety Manual, the same number shall not be assigned.
For Example: First Safety Manual shall be numbered as SM/001.
Where,
| SM | : | Indicate the code of Safety Manual |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of Safety Manual |
ON-SITE EMERGENCY PLAN:
Layout of On-Site Emergency Plan:
Format Considerations:
On-Site Emergency Plan shall contain Header, Footer and Body. “On-Site Emergency Plan” shown in Annexure-VII.
All the points in the On-Site Emergency Plan shall be numbered sequentially and sub paragraph of the On-Site Emergency Plan be also numbered sequentially with an incremental number derived from the heading number.
On-Site Emergency Plan Header shall be covered by a borderline with 3 Line Weight (Width), Specimen of Header of On-Site Emergency Plan shown in Annexure-VII.
Content of the Body:
Front Page: First page body shall have word “On-Site Emergency Plan” font style shall be Times New Roman 65 font in Italic with symbol of Environment Health & Safety as shown in Annexure-VII. Location Name & Address with Logo. First Page Body Part details shown in Annexure-VII.
Index of On-Site Emergency Plan: First Page of On-Site Emergency Plan shall contain “Table of Content” in the form of given table. Header Row of Table shall have gray 25 % Shading Colour.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-of-site-master-file-manuals-and-master-plans/
| S. No. | Description | Page No. |
| – | – | – |
CONTENTS OF ON-SITE EMERGENCY PLAN:
- Approval:
- Safety Policy:
- On-site Emergency Plan Organization Chart:
- Preface:
- Description of the Plant:
- Processes and Probable Emergency Situation on Site:
- General responsibility:
- Systems Available for controlling Emergency Situation:
- General Instructions to Employee:
- After Normal Working hours and Holidays:
- External Emergency services:
- Appendix.
- Conclusion:
- Revision History:
Font Style / Size of Body Contents :
| NAME OF CONTENT | FONT SIZE |
| BODY FIRST PAGE: | |
| On-Site Emergency Pan | 65 Normal & Bold (Italic) in Red Colour |
| Location Name | 16 Capital & Bold In Red Colour |
| Dosage form Description | 12 Normal & Bold in Blue Colour |
| Location Address | 12 Normal & Bold in Black Colour |
Numbering System of On-Site Emergency Plan:
On-Site Emergency Plan shall contain Eight Alphanumeric Characters (Four Alphabets, Three Numeric and One Separators). On-Site Emergency Plan shall be assigned with a Unique Number, once number is allotted to On-Site Emergency Plan, the same number shall not be assigned.
For Example: First On-Site Emergency Plan shall be numbered as OSEP/001.
| OSEP | : | Indicate the code of On-Site Emergency Plan |
| ‘/’ | : | Is Separator |
| 001 | : | Indicate the Serial Number of On-Site Emergency Plan |
GENERATION, ISSUANCE, CONTROL AND RETRIEVAL OF SMF, TRAINING MANUAL, QUALITY MANUAL, VMP, CVMP & SAFETY MANUAL:
New & updated Document shall be generated by QA / Operating Department only.
New Document shall be stamped as “MASTER COPY” in Red ink in Right Upper Corner of the page (on all the pages except Front Page).
Photocopy of this Hard Master Copy shall not be used for any purpose.
Stamp all pages as “CONTROLLED COPY” (Except the Front Page) in Green ink on the Right Upper Corner of the page in Original Print out.
Controlled copy shall be issued to require Department by QA only.
Copy for Reference:
In case, if any Documents is submitted to the external agencies (i.e. Regulatory, Customers / Partners etc.) a Colored Printout from the password protected System shall be taken by QA and shall be stamped as “UNCONTROLLED COPY” in Violet ink on left corner of the page below footer.
If Soft Copy of the Document is forwarded it shall be submitted as “Read only” protected PDF format / Scan Copy.
A Master List of Documents shall be maintained by QA Department as per Format provide in current version of SOP Documentation & Data Control. It contains the list of Documents of Plant & shall be separate.
The list of Documents shall be updated by QA Once in a Year or whenever required, manual corrections shall not be allowed in this list.
REVISION OF SMF, TRAINING MANUAL, QUALITY MANUAL, VMP, CVMP & SAFETY MANUAL:
Any change in Document shall be done only after Authorization of “Change Control”.
The “Change Control” form shall be issued by QA after recommendation of QA Head. After receiving filled Change Control from initiating Department, QA Head shall approve the proposal on the basis of satisfactory scientific rationale.
After Approval of Change Control QA shall incorporate the changes in soft copy. The Head QA shall approve the revised Documents in hard copy.
Previous Master Copy shall be stamped as “OBSOLETE COPY” and Soft Copy of obsolete Documents shall be stored in a separate folder.
The approved revised hard copy of Document shall be treated as “MASTER COPY”.
Revision Period:
| S. No. | Document | Revision Period |
| 1 | Site Master File | Two Years* |
| 2 | Training Manual | Two Years* |
| 3 | Quality Manual | Two Years* |
| 4 | Validation Master Plan | Two Years* |
| 5 | Cleaning Validation Master Plan | Two Years* |
| 6 | Safety Manual | Two Years* |
| 7 | On-Site Emergency Plan | Two Years* |
DESTRUCTION OF SMF, TRAINING MANUAL, QUALITY MANUAL, VMP, CVMP, SAFETY MANUAL & ON-SITE EMERGENCY PLAN:
QA Officer / Executive shall destroy the retrieved Controlled Copy of Document.
QA Officer / Executive shall destroy the Document with the help of Shredder.
REFERENCES:
- PIC/S Guidelines “Explanatory Notes For Pharmaceutical M1anufacturers on the Preparation of a Site Master File” PE 008-04 (1 Annex)-1 January 2011.
- PIC/S Guidelines “Validation Master Plan Installation and Operational Qualification Non-Sterile Process Validation Cleaning Validation” PI 006-3 25 September 2007.
- A WHO guide to good manufacturing practices requirements. Part 2: Validation, World Health Organization, Geneva, 1997 (WHO/VSQ/97.02).
- A WHO Guide to Good Manufacturing Practice (GMP) Requirement Part-3 Training.
ANNEXURES:
| ANNEXURE No. | ANNEXURE TITLE | FORMAT No. |
| Annexure-I | Site Master File | QA-002/F01-00 |
| Annexure-II | Training Manual | QA-002/F02-00 |
| Annexure-III | Quality Manual | QA-002/F03-00 |
| Annexure-IV | Validation Master Plan | QA-002/F04-00 |
| Annexure-V | Cleaning Validation Master Plan | QA-002/F05-00 |
| Annexure-VI | Safety Manual | QA-002/F06-00 |
| Annexure-VII | On-Site Emergency Plan | QA-002/F07-00 |
DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Quality Control |
| Controlled Copy No. 03 | : | Head Engineering |
| Controlled Copy No. 04 | : | Head Production |
| Controlled Copy No. 05 | : | Head EHS |
| Master Copy | : | Quality Assurance |
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| Ltd. | : | Limited |
| QA | : | Quality Assurance |
| IT | : | Information Technology |
| SMF | : | Site Master File |
| OHP | : | Over Head Projector |
| GSM | : | Grammage per square meter |
| GMP | : | Good Manufacturing Practices |
| EU | : | European Union |
| EEA | : | European Economic Area |
| USA | : | United State of America |
| PAT | : | Process Analytical Technology |
| HVAC | : | Heating Ventilation & Air Conditioning |
| AHUs | : | Air Handling Units |
| QRM | : | Quality Risk Management |
| API | : | Active Pharmaceutical Ingredients |
| PLCs | : | Programmable Logic Controllers |
| DQ | : | Design Qualification |
| IQ | : | Installation Qualification |
| OQ | : | Operation Qualification |
| PQ | : | Performance Qualification |
| GLP | : | Good Laboratory Practices |
| : | Portable Documents Format | |
| VMP | : | Validation Master Plan |
| AQ | : | Area Qualification |
| EQ | : | Equipment Qualification |
| CIP | : | Clean-in-Place |
| EHS | : | Environment Health & Safety |
| PPE | : | Personnel Protective Equipment |
| URS FAT SAT | : : : | User Requirement Specification Factory Acceptance Test Site Acceptance test |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | Not Applicable | New SOP | To be filled manual |
ANNEXURE – I
ANNEXURE – II
ANNEXURE -III
ANNEXURE -IV
ANNEXURE – V

ANNEXURE – VI
ANNEXURE- VII
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/preparation-of-site-master-file-manuals-and-master-plans/



