- PROCEDURE:
- General Instructions
- Ensure that the MSDS (Material Safety Data Sheet) of the respective official material is available in the laboratory and all the precautions provided in them are followed.
- Standard Solutions for Qualitative Analysis:
- This procedure is not applicable for solutions from which quantitative parameters such as “area, absorbance, RSD (Relative Standard Deviation) etc.” are measured.
- Prepare a standard solution (Reference standard, in-house reference standard, working standard, impurity standard etc.), which are used for qualitative system suitability parameters such as “resolution factor, capacity factor, tailing factor/symmetrical factor, theoretical plates, etc.,” are measured as per relevant test procedure and shall not be maintained the stock verification.
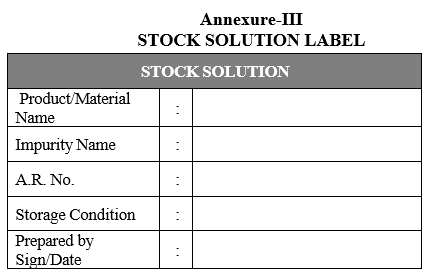
- Store the standard solutions in tightly closed bottles or in sealed vials at a temperature between 2°C and 8°C, unless otherwise specified. Label these bottles/vials using the label as per Format-III.
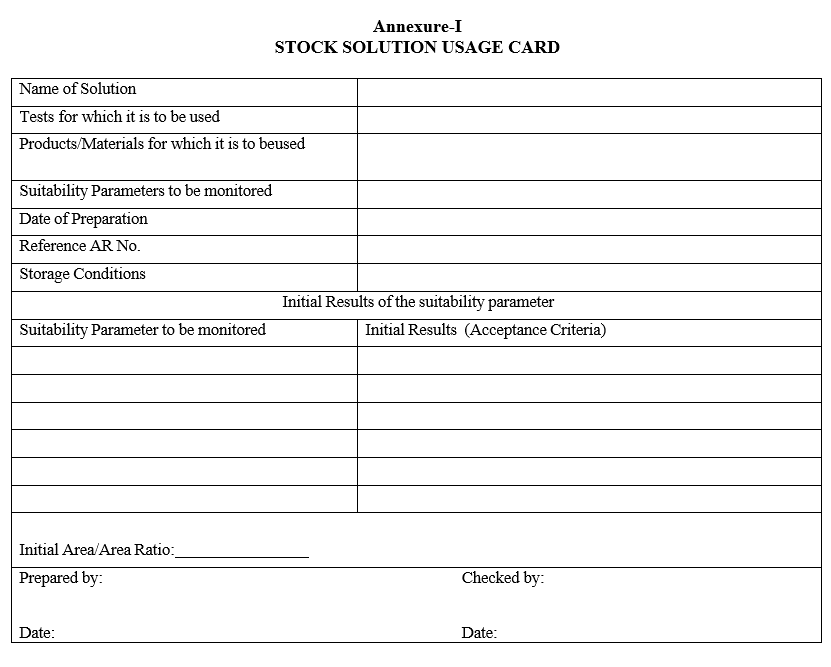
- Prepare and allot the usage card as given in Format-I and fill the details accordingly.
- The system suitability solution can be used until the area or area ratio difference of the principal peak between initial analysis and current analysis is within ± 20%.
- The area difference shall be calculated as follows

- The solution shall be used as long as it is clear, free from turbidity and the solution complies within the acceptance criteria of analytical parameters.
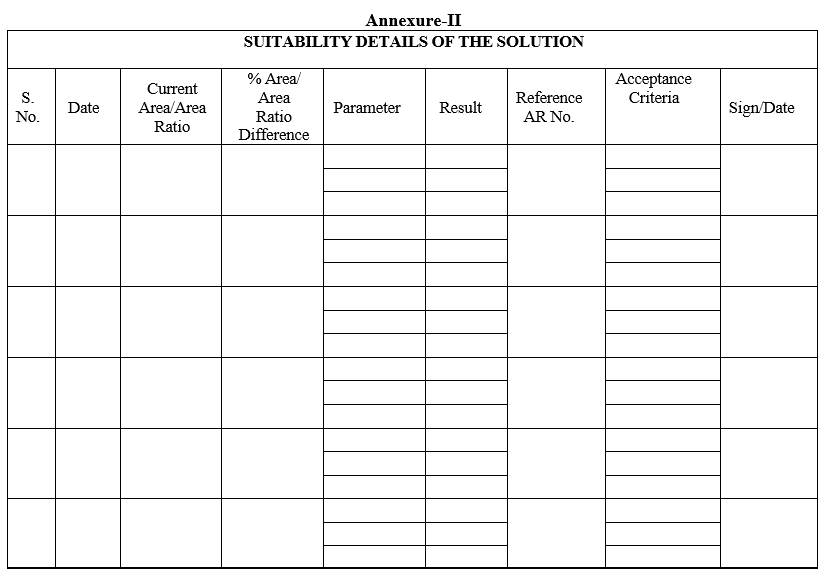
- Whenever usage of solution, enter the details of observed parameters, as given in Format-II.
- The usage is valid only if the observed parameter values are within the acceptance criteria. Else discard the solution.
- Destruction procedure of solutions: Deface the label and send the solutions to SHE dept, destruction shall be followed as per the SOP.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Stock Solution Usage Card |
| Annexure-II | Suitability details of the solution |
| Annexure-III | Stock solution Label |
Annexure-I
STOCK SOLUTION USAGE CARD
Annexure-II
SUITABILITY DETAILS OF THE SOLUTION
Annexure-III
STOCK SOLUTION LABEL