- PROCEDURE:
- Characterization and Qualification of Working Standard:
- Select the current approved batch of respective raw material which meets the specification.
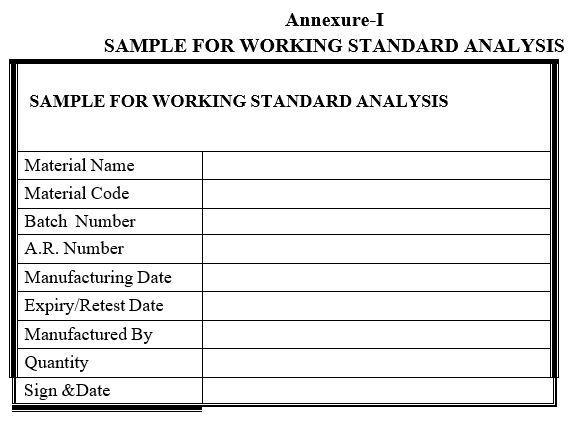
- Get the approved required quantity of raw material from the raw material stores and label it as sample for working standard analysis.
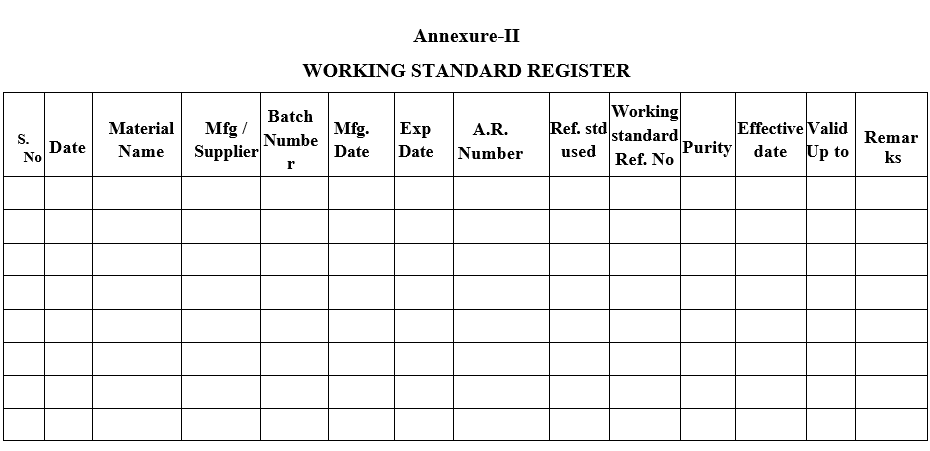
- Registration of working standards shall be done as per Format-II and generate A.R Number as per Sop.
- Allot a Reference number for each working standard.
- The Reference number consists of nine characters i.e. “WSYY001”
Where
The first two characters “WS” refer for working standard
YY for last two digits of current Year
001 for S. No that starts from 001,002 and so on.
E.g. – WS25001 is the first working standard made in the year 2025.
- Always use reference standards for comparison.
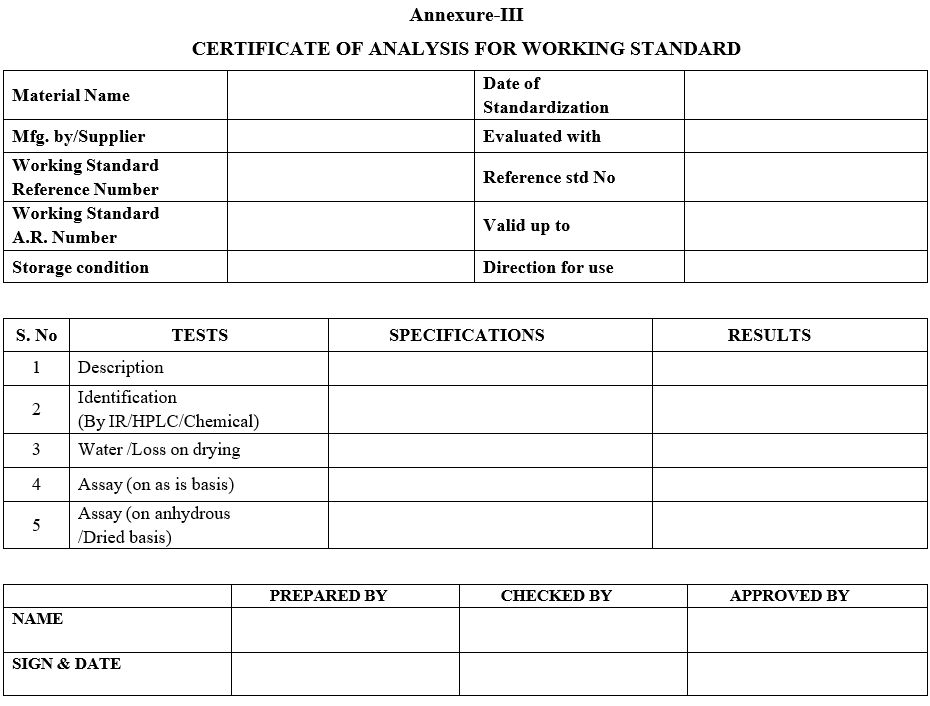
- Analyze the sample for description, identification by IR/HPLC/Chemical, moisture content / LOD, Assay.
- Perform Assay, moisture content/LOD in duplicate, as per the procedure given in respective standard test procedure and report the average value.
- The overall % RSD of the three measurements (Two assay measurements obtained during the qualification of API) and the assay value obtained at the time of batch release of the API should be NMT 3%.
- The results of approved API before and after to standardization should be comparable for Moisture Content/LOD.
- Chemist shall prepare work sheets as per SOP and enter the data in work Sheet and completed work sheet shall be handed over to Section in charge.
- Section In charge or his designee shall ensure that all the tests are performed as per the standard test procedure and results are reported as per the specification, and then he or his designee shall prepare COA as per Format-III.
- Shelf life and storage conditions:
- The shelf life of the working standard shall be one year from the date of standardization.
- If the expiry/retest date of the raw material used for standardization is less than one year.
- Then the same shall be considered as shelf life.
- The storage conditions of the working standard shall be decided based on the Manufacturer COA If the manufacturer COA does not specify storage condition then it should be stored at room temperature.
- The details of shelf life and storage conditions shall be entered in the format.
- Handling:
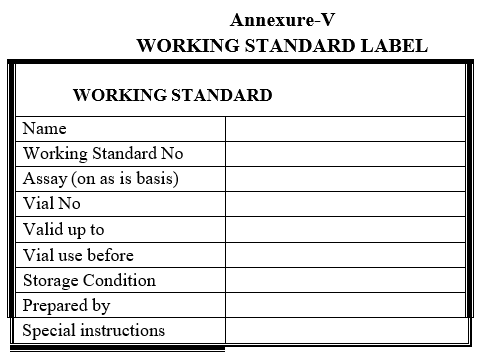
- Transfer the working standards into 15 amber colored glass vials with screw cap under Laminar air flow, seal the cap with parafilm and label with working standard label as given in Format-V.
- Out of 15 vials, 1 vial is odd vial, 12 vials for 12 months and 2 vials are stand by vials.
- The working standard vials should be replaced on 1 st of every month, the odd vial is used in the month of standardization.
- Store the working standards as per the recommended storage conditions.
- The working standard vial shall not be used beyond the use before date.
- Store the in use working standards in desiccators to prevent moisture absorption.
- Note: When stored at 2°C-8°C or -20°C, allow the standard to attain room temperature before usage.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/standardization-and-storage-of-working-standards-primary-standards-gas-chromatography-standards-and-placebos/
- PrimaryStandards Usage and Storage:
- Primary standards are high purity chemicals.
- Primary standards are used to standardize the volumetric solutions.
- Use A.R/G.R grade chemicals as primary standards.
- Primary standards shall be stored in segregated labeled area (Primary standards).
- Gas Chromatography Internal Standards:
- G.C standards are reagents of high grade purity which are used as standards in G.C Analysis.
- Use A.R grade reagents of purity ≥ 99 %.For quantitative determination use the assay value printed on the label.
- G.C standards shall be stored in a segregated labeled area (G.C standards).
- Placebo:
- Placebos are stored in segregated area labeled as (Placebos).
- Placebo should be labeled as per Format-VI.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Sample for working standard analysis |
| Annexure-II | Working standard register |
| Annexure-III | Certificate of Analysis for working standard |
| Annexure-IV | Assignment of shelf life to working standard |
| Annexure-V | Working standard label |
| Annexure-VI | Placebo label |
- ABBREVIATIONS:
| No. | : | Number |
| LOD | : | Loss on drying |
| IR | : | Infra red |
| HPLC | : | High performance liquid chromatography |
| COA | : | Certificate of analysis |
| GC | : | Gas chromatography |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Annexure-I
SAMPLE FOR WORKING STANDARD ANALYSIS
Annexure-II
WORKING STANDARD REGISTER
Annexure-III
CERTIFICATE OF ANALYSIS FOR WORKING STANDARD
Annexure-IV
ASSIGNMENT OF SHELF LIFE TO WORKING STANDARD

Annexure-V
WORKING STANDARD LABEL
Annexure-VI
PLACEBO LABEL
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/standardization-and-storage-of-working-standards-primary-standards-gas-chromatography-standards-and-placebos/
