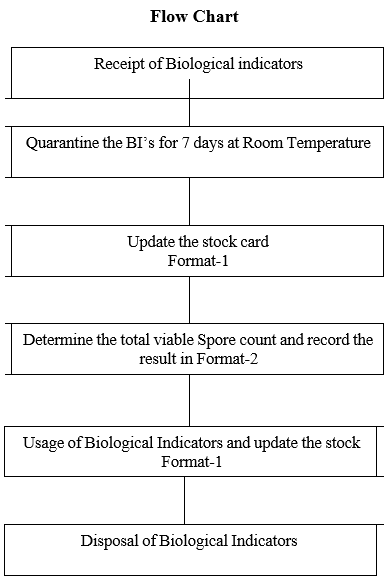
- PROCEDURE FOR RECEIPT, ENUMERATION, USAGE AND DISPOSAL OF BIOLOGICAL INDICATORS:
- A biological indicator is a specific type of microorganism that is used to determine the effectiveness of a sterilization process. These indicators are typically highly resistant to sterilization methods, such as endospores from Bacillus pumilus. If the sterilization process is successful, all of the biological indicators will be killed. The presence of any surviving biological indicators indicates that the sterilization process may not have been effective and that there is a risk of contamination.
- Receipt and Storage of Biological Indicators:
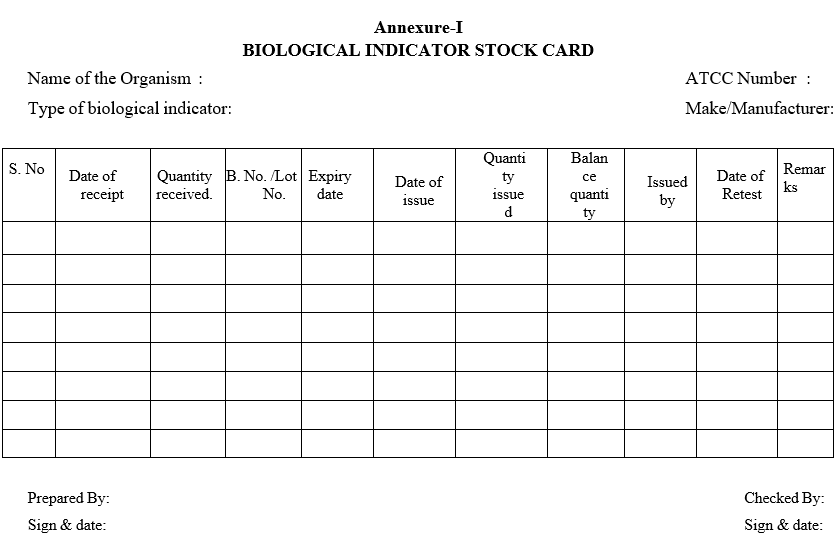
- Upon receipt of biological indicators enter the relevant details as per Format-I.
- Ensure that the certificate of analysis is received along with the lot of biological indicators and check the certificate for the following information.
- Name of the organism along with the ATCC number from which the spores are derived.
- Batch Number / Lot Number
- Total viable spore count or mean population per unit of biological indicator.
- ‘D’ value and the method used to determine ‘D’ value.
- ‘Z’ value
- Expiry date
- Storage specifications.
- Disposal procedure
- Bacteriological medium, which will meet the requirement for growth promoting ability.
- Conditions under which reproducible resistance characteristics are obtained.
- Survival time and kill time under specified conditions.
- Place the biological indicator ampoules / self-contained vials under Quarantine for 7 days at controlled room temperature.
- Check visually for contamination (purple to yellow color) and if found any contaminated unit, discard it.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-enumeration-usage-and-disposal-of-biological-indicators/
NOTE: Purple color (No growth) to Yellow color (growth observed).
NOTE: Store the biological indicators as per manufacturer’s instructions
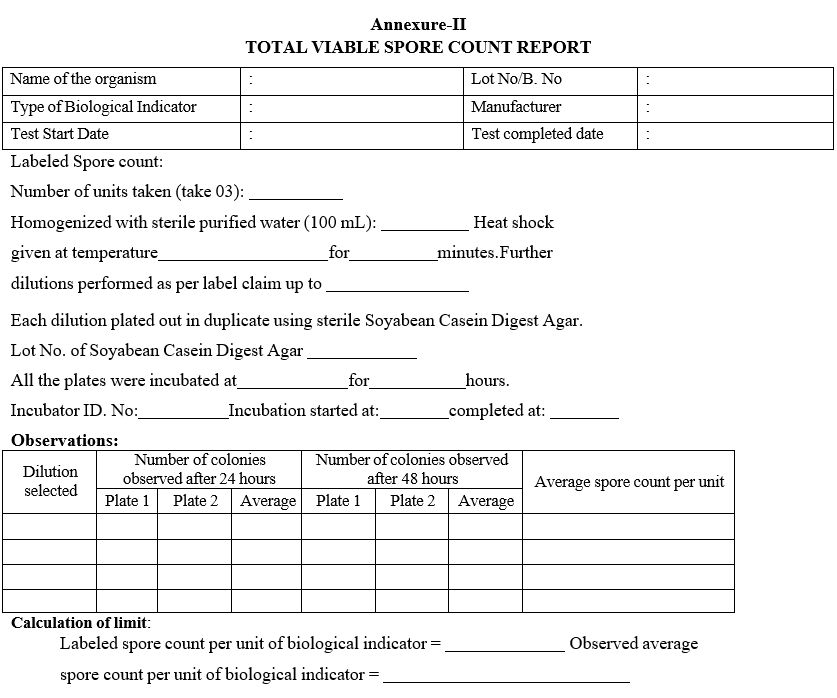
- Determination of Total viable spore count of Biological Indicator for Moist Heat Sterilization:
- Biological indicators, Type–Ampoules:
- Randomly pick 3 ampoules from each Batch received. Under aseptic conditions.
- Pool the contents in a sterile 250 mL conical flask.
- Add pre-sterilized, chilled purified water to make 100-mL and mix for 3 to 5 minutes to achieve a homogeneous suspension, which gives 10 -2 dilution.
- Transfer a 10-mL aliquot to a sterile screw cap tube / cotton plugged tube/capped tube and place the tube in a water bath at 95°C to 100°C for 15 minutes.
- Cool rapidly in an ice water bath (0°C to 4°C).
- Transfer 1-mL aliquot to suitable tube and prepare further10-fold serial dilution in sterile purified water to yield 25 to 250 colonies when plated.
- The serial dilutions are prepared as per the label claim.
- Place 1 mL of each from selected dilution into 2 sterile Petri plates.
- Within 20 minutes, add to each plate 15-20mL of Soyabean Casein Digest Agar Medium, which has been sterilized and cooled to 45°C to 50°C. Mix well and allow to solidifying.
- Incubate the plates in an inverted position at 55°C to 60°C for 48 hours.
NOTE: Avoid drying of plates during incubation by keeping a beaker containing water in the incubator.
- Examine the plates for evidence of contamination with other microorganisms.
- Count the number of colonies after 24 and 48 hours, using the number of colonies after 48 hours to calculate the final results.
NOTE: The test is valid, if the log number of spores per ampoules after 48hours is equal to or greater than the log number of spores after 24hours.
- Calculate the average of the number of viable spores per container.
- Calculate the log of average number of viable spores per container.
- Calculate the lower limit for Total viable spore count by determining the log of the labeled spore count per container and subtracting 0.3 from the value.
- Calculate the upper limit for the Total viable spore count by summing 0.48 to the log value of the labeled spore count per container.
NOTE: Example for calculation of Total viable spore count.
- Labeled spore count per ampoule/strip = 1.2 x 10 6
- Average spore count per ampoule/strip = 2.2 x 10 6
- Lower limit is calculated by subtracting 0.3 from the log value of the Labeled spore count per ampoule = 6.079 – 0.3 = 5.779 (Lower limit)
- Upper limit is calculated by summing 0.48 to the log value of the labeled spore Count per ampoule = 6.079 + 0.48 = 6.559 (Upper limit)
- Log average spore count per ampoule = 6.342
- Biological indicators, Type- Paper Carriers:
- Randomly pick 3 strips from each Batch received.
- Under aseptic conditions remove the strips from the individual envelope.
- Place these strips in 250-mL sterile conical flask containing 100 mL of pre sterilized, chilled purified water and sterile glass beads.
- Vortex until the paper carrier is macerated to achieve a homogeneous suspension.
- Biological indicators, Type – Self- Contained:
- Randomly pick 3 strips from each Batch received. Under aseptic conditions, remove the strips from the individual Containers.
NOTE: Avoid spillage while handling self- contained indicators.
- Place these strips in 250-mL sterile conical flask containing 100 mL of pre sterilized, chilled purified water and sterile glass beads.
- Vortex until the paper carrier is macerated to achieve a homogeneous suspension.
- Determination of Total viable spore count of Biological indicator for Dry Heat Sterilization:
- Biological indicators, Type – Paper Carriers:
- Randomly pick 3 strips from each Batch received, under aseptic conditions, remove the strips from the individual envelope.
- Place these strips in 250-mL sterile conical flask containing 100 mL of pre sterilized, chilled purified water and sterile glass beads.
- Vortex until the paper carrier is macerated to achieve a homogeneous suspension.
- Transfer a 10-mL aliquot to a sterile screw cap tube and place the tube in a water bath at 80°C to 85°C for 10 minutes.
- Cool rapidly in an ice water bath (0°C to 4°C).
- Incubate the plates in an inverted position at 30°C to 35°C for 48 hours.
- Examine the plates for evidence of contamination with other microorganisms.
- Acceptance Criteria:
- The requirements of the test are met if the log average number of viable spores per container is not less than 0.3 log labeled spore count per container and does not exceed the log labeled spore count per container by 0.48.
- Usage of Biological indicators in validation of Sterilization process:
- Issue the tested and approved batch of biological indicators for validation of Sterilization process.
- Update the stock card as per Format-I.
- Retest biological indicator ampoules/ strips / self-contained vials for total viable spore count per unit yearly.
NOTE: Retesting period – Yearly.
- Disposal of Biological indicators:
- Prior to disposal, used biological indicator ampoules/strips/self-contained vials shall be decontaminated at 121°C for 30 minutes as per SOP.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-enumeration-usage-and-disposal-of-biological-indicators/
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Biological indicator stock card |
| Annexure-II | Total viable spore count report |
- ABBREVIATIONS:
| No. | : | Number |
| ATCC | : | American Type Culture Collection |
| SCD | : | Soyabean Casein Digest Agar. |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Flow Chart FOR BI
Annexure-I
BIOLOGICAL INDICATOR STOCK CARD
Annexure-II
TOTAL VIABLESPORE COUNT REPORT
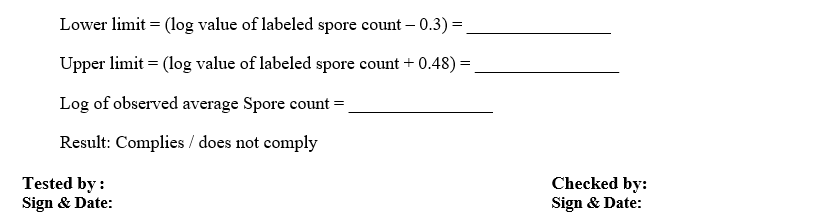
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/receipt-enumeration-usage-and-disposal-of-biological-indicators/

