Labelling on ampoules and vials is crucial for ensuring patient safety and accurate medication use. These tiny containers hold powerful substances, and the label provides essential information for healthcare professionals and patients.
However, the following general points should be followed while labelling & packing:.
Ampoule Packaging Instructions:
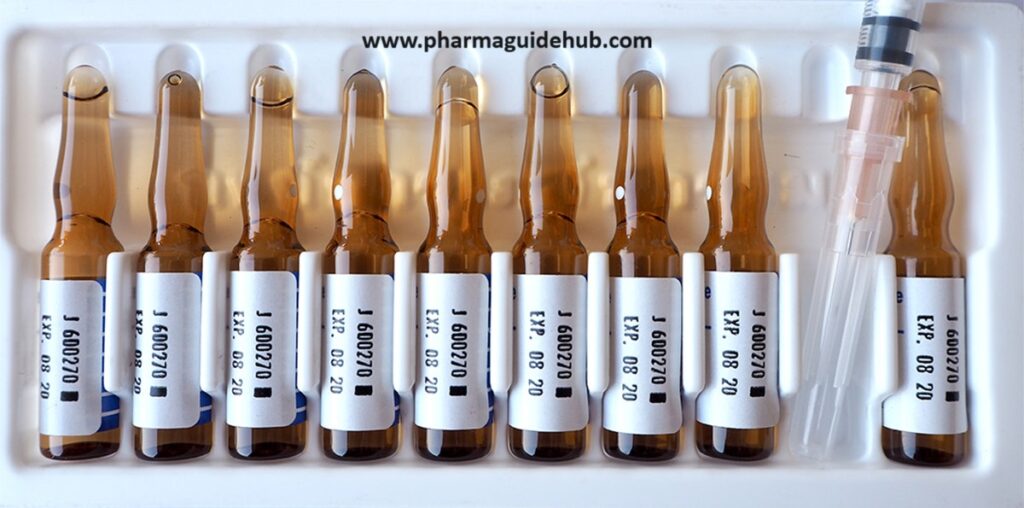
- Materials:
- Ampoules (properly inspected and sterilized)
- Labels (pre-printed with all required information)
- Blister packs (optional)
- Cartons
- Package inserts (if applicable)
- Desiccant packs (if required for moisture control)
- Personnel:
- Trained personnel who understand aseptic techniques (if applicable)
- Quality control personnel
- Procedure:
- Preparation:
- Wash hands thoroughly and follow aseptic procedures if required for the product.
- Ensure the work area is clean and free of contamination.
- Gather all necessary materials.
- Ampoule Inspection:
- Visually inspect each ampoule for cracks, chips, or other defects. Discard any damaged ampoules.
- Verify the ampoules are filled to the correct level.
- Labeling:
- Apply labels to each ampoule according to your labeling procedures. Ensure all required information is present and clearly visible (e.g., drug name, dosage, expiry date, lot number).
- Double-check the labels for accuracy before applying.
- Blister Packing (Optional):
- If using blister packs, carefully place each labeled ampoule in its designated cavity within the blister pack.
- Ensure the ampoules are securely held in place and protected from damage.
- Cartoning:
- Place the ampoules (or blister packs) into the designated cartons.
- Include any required package inserts within the carton.
- If necessary, add desiccant packs for moisture control.
- Sealing and Labeling:
- Securely seal the cartons according to your packaging specifications.
- Apply outer labels to the cartons with relevant information (e.g., product name, quantity, storage conditions).
- Quality Control:
- Conduct a final inspection of the packaged ampoules to ensure they meet all quality standards.
- Document the packaging process according to your quality control procedures.
- Additional Considerations:
- Storage: Store the packaged ampoules in a clean, dry, and cool environment according to the product specifications.
- Shipping: Follow proper shipping regulations for pharmaceutical products, considering factors like temperature control and fragility.
- Documentation: Maintain complete documentation of the packaging process, including batch numbers, expiry dates, and any deviations from procedures.
Another General instructions for labelling & packing should be followed for each product:
- A request slip is made to the stores to issue approved packing material.
- Each ampoule / vial is cleaned with moist cloth or washed as required.
- Check packing material for correct batch No., Mfg.date, exp. date & MRP.
- Paste labels on each ampoule / Vial, remove extra gum, let them dry.
- Polish them with wet cloth followed by dry cloth.
- Re label the ampoule / vials on which label is not straight or torn.
- Pack ampoules / vials in their respective box/ multiple box along with dropper etc.
- Put adhesive tap and outer label on the box and put it in shipper.
- Affix clip and strip on shipper.
- All left over packing material should be reconcile and send back to warehouse with excess material return note.


