OBJECTIVE:
To lay down a procedure forSampling and Testing of Packing Material.
SCOPE:
This SOP is applicable for Sampling and Testing of Packing Material of Quality Control Department at {Company Name} {Location}.
RESPONSIBILITY:
All entrants – To follow the instruction as per SOP
QC Head – Review, technical correction, training and monitoring of SOP.
QA Head – Approval and implementation of SOP.
- ACCOUNTABILITY:
QA Head shall be accountable for implementation of SOP.
PROCEDURE:
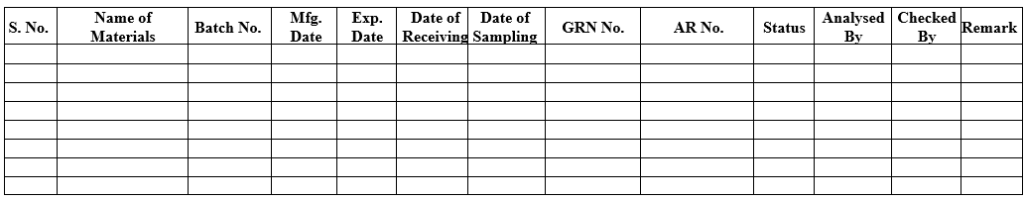
- QC Officer shall maintain all details in packing material inward register from GRN as per Annexure-IV
- QC Officer shall plan the sampling activity accordingly and QC Executive shall issue the under test and sampled labels to QC Officer.
- QC Officer shall inform to packing material store department about sampling.
- Take 100% sample for primary packing material.
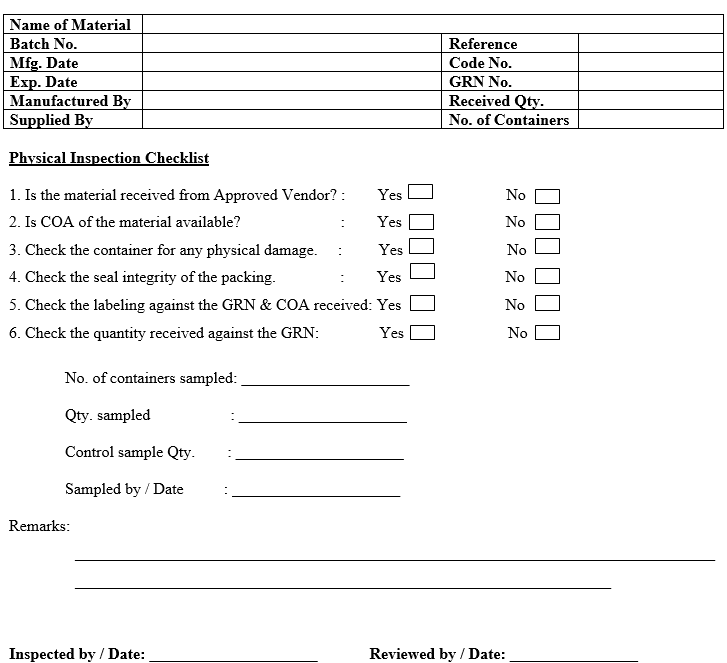
- QC Officer shall verify the GRN details against the detail mentioned n containers/ packs and record the observations in packing material inspection report as per Annexure-III
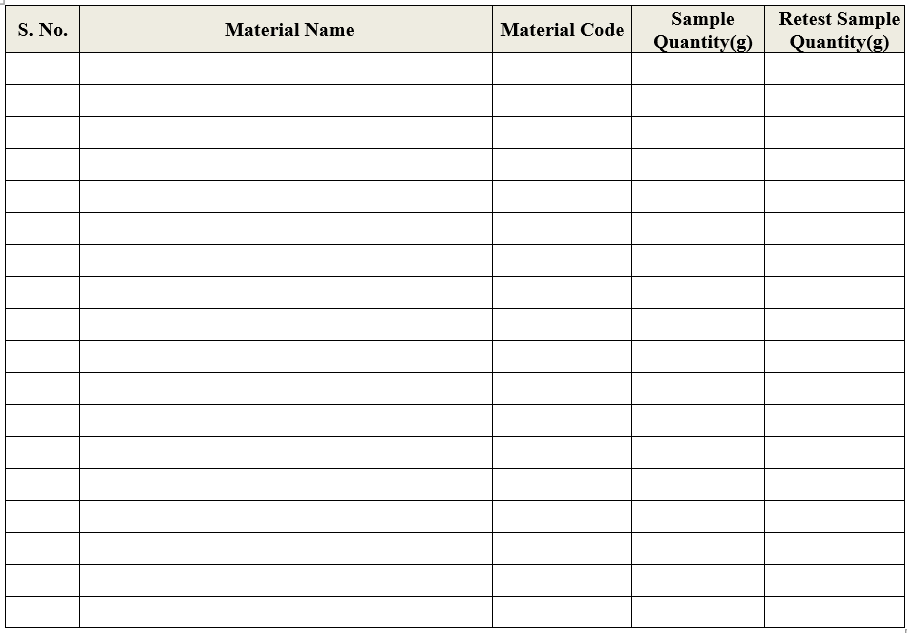
- QC Officer shall take the sample quantity as per respective specification as per Annexure-II

- Analysis (Physical parameter) of tertiary packing material shall be performed with 5 samples from each consignment in store and only one sample shall be carried out in QC for other tests.
- Take the sample of primary packing material under LAF.
- Check the pressure differential of LAF (if applicable).
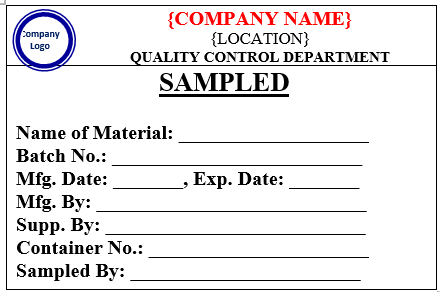
- Affix a label ‘SAMPLED’ to identify the container/packs from which the sample is drawnas per Annexure-I
- Affix the label ‘UNDER TEST’ to the container/pack.
- After collecting the sample from store, QC Officer shall sign with date in sampled by/date column in packing material inward register.
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE | NAME OF ANNEXURE |
| Annexure-I | List of pack materials sample quantity for analysis |
| Annexure-II | Sampled Label |
| Annexure-III | Packing Material Inspection Report |
| Annexure-IV | Packing Material Inward Register |
DISTRIBUTION:
| Controlled Copy No. 01 | : | Manager Quality Assurance |
| Controlled Copy No. 02 | : | Manager Quality Control |
| Master Copy | : | Quality Assurance Department |
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| QC | : | Quality Control |
| GRN | : | Good Receipt Note |
| LAF | : | Laminar Air Flow |
| CFR | : | Code of Federal Regulation |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable |
ANNEXURE-I
LIST OF PACK MATERIALS SAMPLE QUANTITY FOR ANALYSIS
ANNEXURE-II
SAMPLED LABEL
ANNEXURE-III
PACKING MATERIAL INSPECTION REPORT
ANNEXURE- IV
PACKING MATERIAL INWARD REGISTER