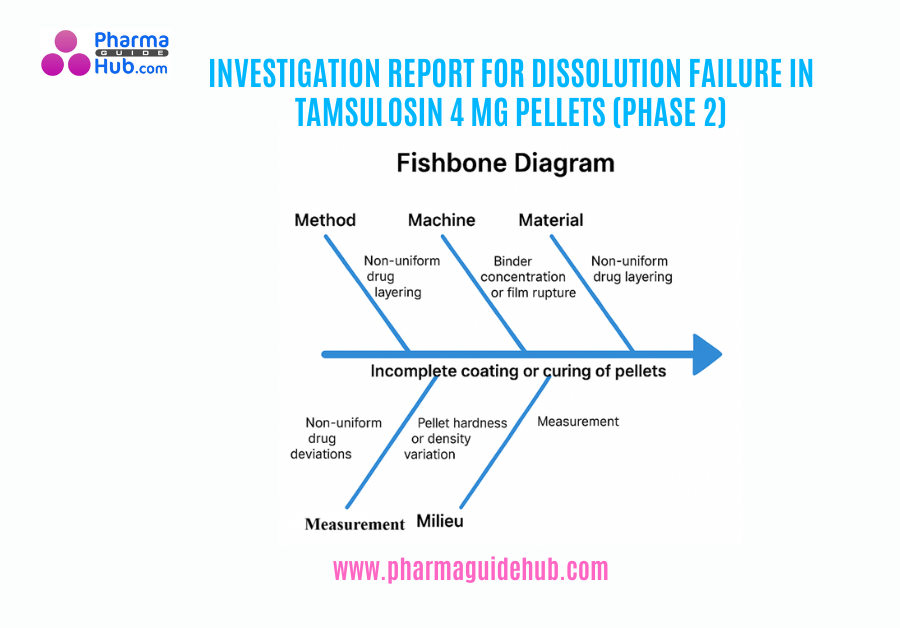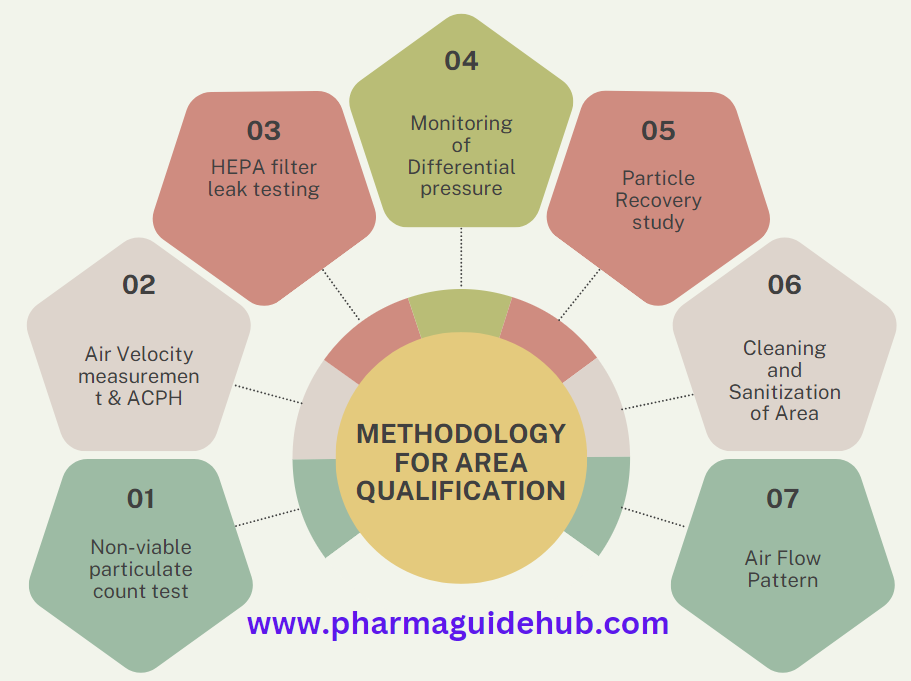
Area Qualification in the pharmaceutical industry is a critical process that ensures that specific areas within a manufacturing facility are suitable for their intended use. It involves a series of tests and assessments to verify that the area meets the required standards for cleanliness, temperature, humidity, air quality, and other relevant parameters.
Methodology: Involves various tests and measurements, including:
Cleaning and sanitization verification
Particle counting
Air velocity and flow rate
HEPA filter integrity testing
Differential pressure monitoring
Temperature and humidity control
Microbial contamination assessment