- OBJECTIVE:
To lay down a procedure for Movement of Batch Documents.
- SCOPE:
This SOP is applicable for Movement of Batch Documents at {Company Name} {Company Location}.
- RESPONSIBILITY:
QA, QC, Production & Warehouse- Follow the instruction as per procedure.
QA Head –Training, approval & implementation of SOP.
- ACCOUNTABILITY:
QA Head
- PROCEDURE:
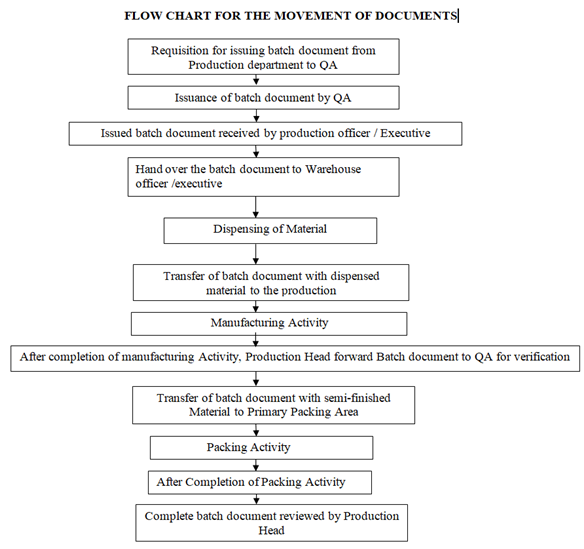
- On receipt of requisition for issuing of documents from production department, QA shall issue the batch document (BMR & BPR) to production.
- Production officer/executive shall review the issued batch document and handover to Raw material warehouse officer/ Executive for dispensing of raw materials & Packing material warehouse officer/ Executive for dispensing of packing materials.
- On the basis of material Requisition, Warehouse officer/executive shall check all the materials physically and start the dispensing activity.
- After the completion of dispensing, Warehouse Officer/Executive return Batch document along with dispensed material to the production Officer/Executive.
- After Receiving the Batch Documents, Production Officer /Executive starts the manufacturing Activity.
- After the completion of manufacturing process, production officer/executive forwards the batch document to production head.
- Production Head shall check the reconciliation record in BMR and forward to QA Officer/Executive for verification.
- After reviewing the Batch manufacturing Record, Batch document with semi-finished material Transferred to Primary packing Area.
- After the completion of packing process, production officer/executive forwards the batch document to production head.
- Production Head shall check the reconciliation record in BPR and forward to QA Officer/Executive for verification.
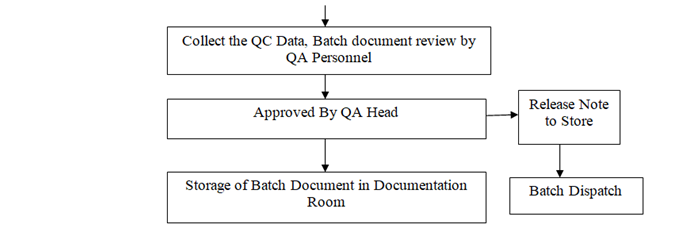
- QA officer/executive shall collect the respective QC data and review the batch documents.
- QA officer/executive forwards the batch document to QA head for final approval & release of batch.
- QA Head verify the batch document and put the signature on BPR & issues a release note to Warehouse Head.
- After releasing the batch, batch document shall be stored in the documentation room. Batch document (BMR & BPR) shall be moved along with material from dispensing to final process.
- Movement of batch documents shall be as per following flow chart.
- REFERENCES:
Not applicable
- ANNEXURES:
Not applicable
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Production |
| Controlled Copy No. 03 | : | Head Warehouse |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| QA | : | Quality assurance |
| SOP | : | Standard operating procedure |
| BMR | : | Batch Manufacturing Record |
| BPR | : | Batch Packing Record |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be recorded manual |