OBJECTIVE:
To lay down a Procedure for Documentation and Data Control.
SCOPE:
This SOP is applicable for Issuance, Control, Distribution, Revision, Archival and Destruction of Documents / Data relating to all Departments. This SOP is applicable to Manufacturing Locations of {Company Name} {Company Location}.
RESPONSIBILITY:
- QA Officer/ Executive shall be responsible for issuance, retrieval and destruction of documents.
- All Concerned department shall follow the instruction as per SOP.
- QA Sr. Executive / Executive shall be responsible for Review and technical correction of SOP.
ACCOUNTABILITY:
Head QA shall be accountable for approval, Training and implementation of SOP.
PROCEDURE:
- ISSUANCE AND RETRIEVAL OF STANDARD OPERATING PROCEDURES:
- All the departments shall submit their signed copy of standard operating procedures to QA department along with the respective training records.
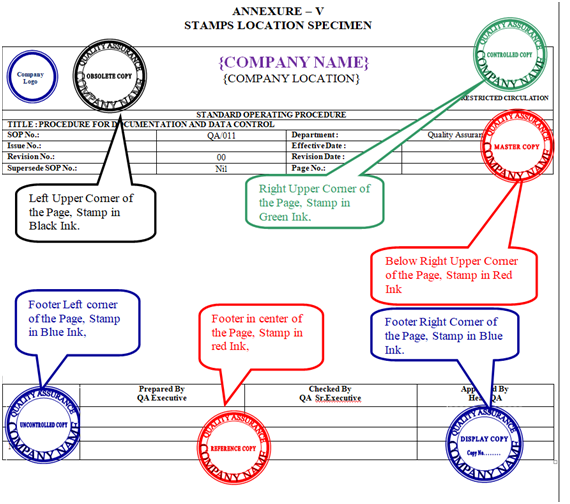
- A Specimen of all Stamps Location is shown in Annexure-V.
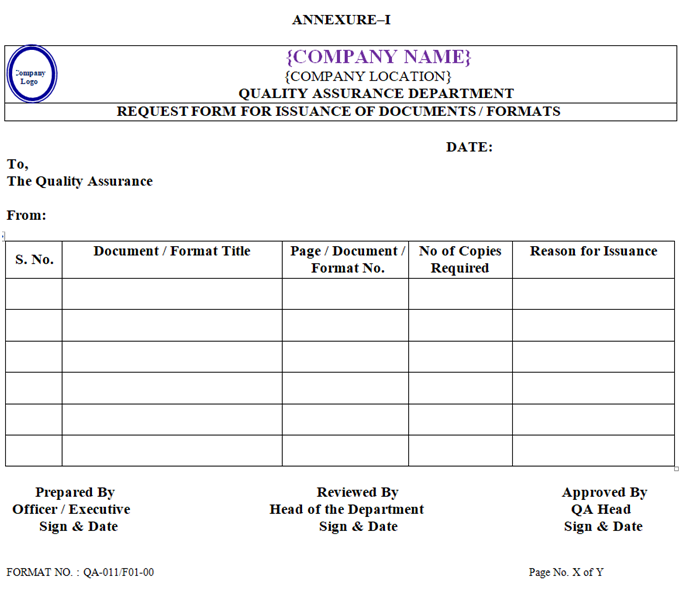
- As per the requirement, requisition for issuance of documents as per Annexure-I shall be raised by the concerned department officer/executive and submitted to QA officer/executive after signature of concern department head for issuance of standard operating procedure.
- After receiving the requisition, QA officer/executive shall take the required number of photocopies of the master copy and issue to concerned departments.
- After any revision of a SOP, QA officer/executive shall withdraw all the issued controlled copies from respective department & destroy prior to effective of revised SOPs.
- These withdrawn SOPs shall be destroyed by shredding.
Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/
- ISSUANCE AND RETRIEVAL OF PRINTED LOGBOOKS, FORMATS IN THE FORM OF BOUND BOOKS:
- Concerned department head shall be responsible for the procurement of all required quality documents, like printed logbooks & formats in the form of bound books of the respective area as per the Annexure mentioned in the respective SOP.
- Concerned department head shall raise the order to outside printing press to make the printed logbooks & formats in the form of bound books by giving specimen format along with order.
- After receiving logbooks & bound books from the printing press, concerned department head shall check for the correctness of formats against master format & then hand over it to QA officer/executive.
- As per the requirement, requisition for issuance of documents as per Annexure-I shall be raised by the concerned department officer/executive and submit it to QA officer/executive after signature of concern department head for the issuance of printed log books & bound book.
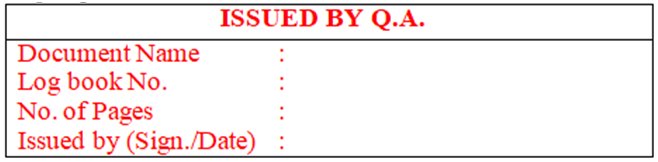
- After receiving the requisition, QA officer/executive shall issue the required number of logbooks, bound book & registers with log book number by putting red colour stamp along with signature & date. Stamp shall be affixed inside of the front cover page on top left side of logbooks & bound books as per given below details:
- Log book number shall be assigned as:
XX-LB-YYY
The 1st and 2nd character ‘XX’ indicates the department code.
The 3rd and 6th character ‘-’ is a hyphen.
The 4th & 5th characters ‘LB’ stands for log book.
The 7th, 8th and 9th characters ‘YYY’ indicates serial No. assigned to each log book
For Example: The first Log book of QA department shall be QA-LB-001.
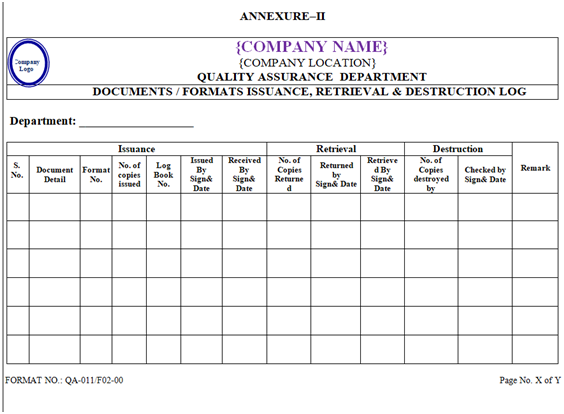
- Entry of issued documents as well as retrieved & destructed documents shall be made simultaneously in the document issue & retrieval register as per Annexure-II along with signature & date of QA officer/executive.
Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/
- All Master Documents, official documents and controlled documents shall be signed by Blue Ink Ball Point Pen.
- In process by IPQA shall be signed by Black Ink Ball Point Pen.
- ISSUANCE AND RETRIEVAL OF FORMAT, ANALYTICAL WORKSHEET & PROTOCOL FOR RECORDING:
- As per the requirement, requisition for issuance of documents as per Annexure-I shall be raised by the concerned department officer/executive and submit it to QA officer/executive after signature of concern department head for the issuance of format, analytical worksheet, protocol.
- After receiving the requisition, QA officer/executive shall take the required number of photocopies from the master copy.
- QA officer/executive shall issue the photocopies by putting “CONTROLLED COPY” stamp in green ink in right upper corner on all pages.
- QA officer/executive shall also put “Issued by QA sign & Date” stamp in green ink on top left-hand corner only on front pages along with sign & date.
- “Issued by QA sign & Date” stamp shall not be affixed on those documents which is already having issued by column but “CONTROLLED COPY” shall be affixed in green ink in right upper corner on all pages of that particular documents.
- Entries of issuance shall be made simultaneously in respective format/register as per Annexure-II along with the signature of the issued by QA officer/executive and the receiving person. Similarly the entries of the retrieval of the completed document shall be made in the respective column of the format/register along with the signature of the returning person and the receiving QA officer/executive.
- Documents for the coming month shall be issued to concerned departments only after returning the previous month’s completed documents to QA officer/executive. Excess or shortage of formats shall be mentioned with reason (given by concerned department) and shall be destroyed by QA officer/executive.
- Analytical worksheet & protocol for semi-finished & finished product analysis shall be given by QA officer/executive along with semi-finished & finished product analysis requisition slip and no need of document request slip required for issuance of respective analytical worksheet & protocol.
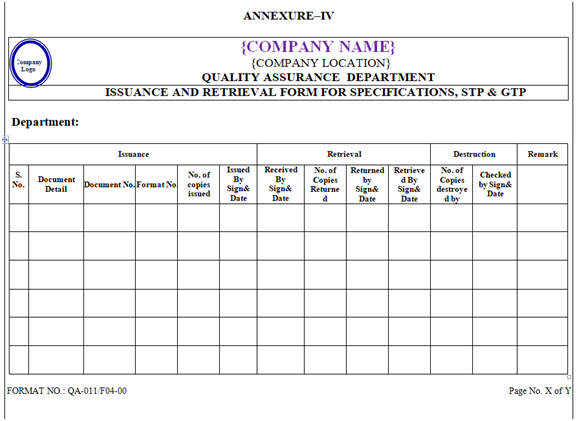
- ISSUANCE AND RETRIEVAL OF SPECIFICATIONS, STANDARD TEST PROCEDURE & GENERAL TEST PROCEDURE:
- As per the requirement, requisition for issuance of documents (as per Annexure-I) shall be raised by the concerned department officer/executive and submit it to QA officer/executive after signature of concern department head for the issuance of specifications, standard test procedure & general test procedure.
- After receiving the requisition, QA officer/executive shall take the required number of photocopies of the required specifications, standard test procedure & general test procedure from the master copy.
- QA officer/executive shall issue the photocopies by putting “CONTROLLED COPY” stamp in green ink in right upper corner on all pages & also put “Issued By QA sign & Date” stamp in green ink on the top left hand corner on front page only of the respective documents along with his sign. /Date.
- At the time of issuance, QA officer/executive shall enter a copy number manually in the space provided to required photocopies for the distribution. (Copy No. shall not be assigned to the master copy).
- Entries of issuance shall be made simultaneously in respective issuance record as per Annexure-IV along with the signature of the issued by QA officer/executive and the receiving person. Similarly the entries of the retrieval of issued control copy (supersedes copy of revised specification/STP/GTP at the time of revision) shall be made in the respective column as per Annexure-IV with the signature of the returning person and the receiving QA officer/executive.
- ISSUANCE AND RETRIEVAL OF BMR & BPR:
- Issuance and retrieval of batch manufacturing records and batch packing records shall be done as per SOP Title “Preparation of Batch Manufacturing and Batch Packing Records Preparation of SOP No. QA/005.
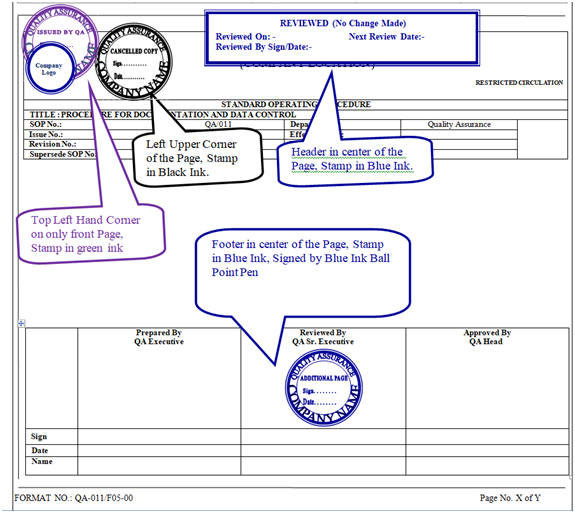
- When any controlled document is required to distribute outside the premises to any regulatory agencies/customer, the photocopy of the master copy of that particular document shall be stamped as “UNCONTROLLED COPY” in blue ink on left corner of the page below footer of all pages.
- ISSUANCE OF ADDITIONAL PAGE
- For Additional copies of documents, concern department shall send the request to QA as per internal procedure of organization.
- QA officer/executive shall issue the Additional page by putting “CONTROLLED COPY” stamp in green ink in the right upper corner on all pages.
- QA officer/executive shall also put “ADDITIONAL PAGE SIGN & DATE” stamp in blue ink at footer in center on all pages along with sign & date.

Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/
- ISSUANCE / RETRIEVAL OF REVIEWED REVALIDATE DOCUMENTS
- Concerned department shall fill the format Request for revalidation of SOP’S and submit to QA Head after signature of concern department head for approval as per Annexure-VII of SOP No. QA/001.
- As per the requirement, requisition for issuance of documents as per Annexure-I shall be raised by the concerned department officer/executive and submit it to QA officer/executive after signature of concern department head for issuance of document.Based on approved request for revalidation of sop’s QA Officer/ Executive shall put “Reviewed (No Change Made)” stamp in blue ink at Header in center of all pages in master documents with sign & date and procedure for revalidation of documents and SOP’s shall be mentioned in SOP No. QA/001.After getting the requisition, QA officer/executive shall withdraw all the issued controlled copies from respective department & destroyed by shredding and record (as per SOP No.: QA/001) prior to effective of Reviewed Revalidate of documents.
- QA officer/executive shall take the required number of photocopies of the master copy and issue to concerned departments as per Annexure-IV and SOP No. QA/001.
- OBSOLETE DOCUMEENT:
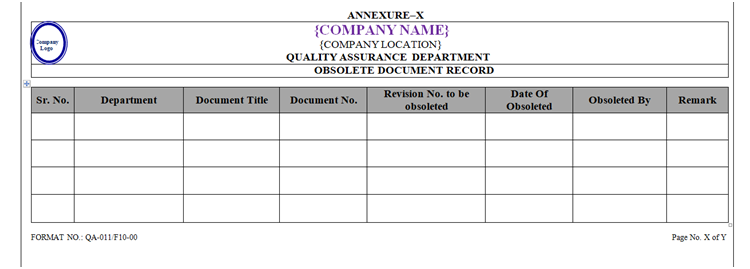
- The document shall be obsoleted as per Annexure-X (Obsolete Document Record).
- STORAGE OF DOCUMENT
- Documents shall be stored in the QA document room.
- Following documents shall be store in the QA document room.
- Master documents, obsolete documents like MFR, BMR, BPR, SOP, validation protocols, change control forms, CAPA forms, Incident/Deviation Forms, analytical protocols, formats & filled completed documents from all the departments which includes BMR, BPR, validation reports, logbooks and cleaning records, analytical reports, annual product quality review, vendor audit report, training records, customer complaint register.
- All record shall be stored in QA document room under lock and key to minimize the potential for damage or deterioration and shall be readily accessible and retrievable upon request.
- The key shall be available with QA officer/executive.
- All the documents shall be stored in the defined rack in QA document room.
- Rack No. to be defined as (Rack No.: 01, 02, 03………). A display record shall be maintained on the rack as per Annexure-VI.
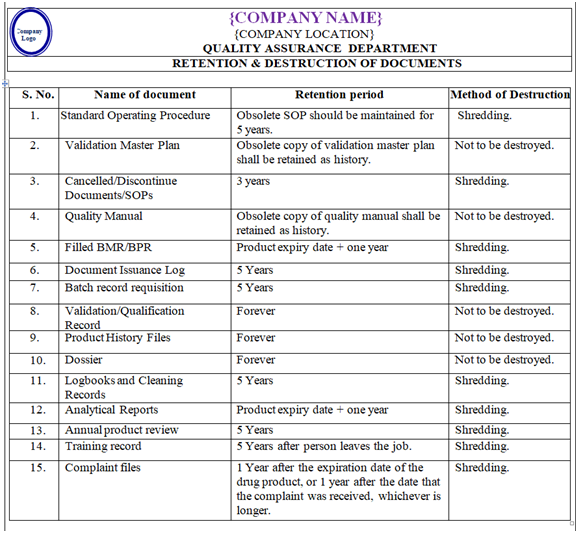
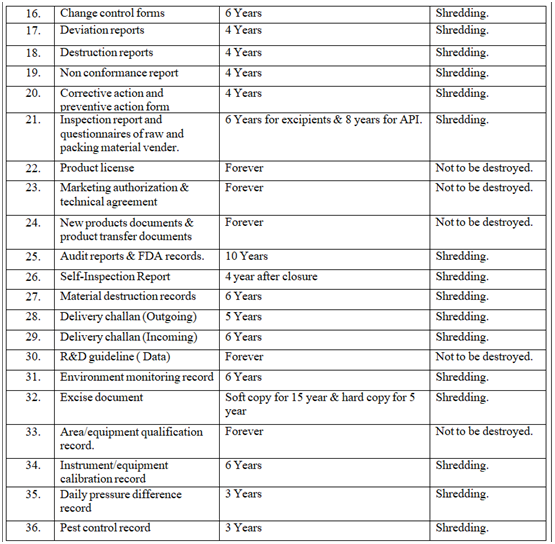
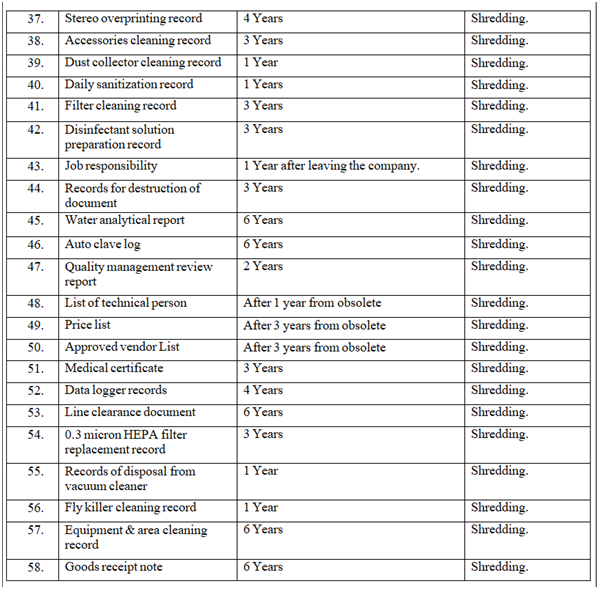
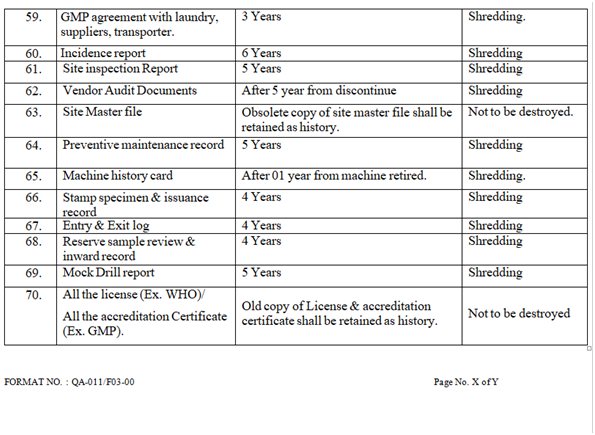
- RETENTION & DESTRUCTION OF DOCUMENTS:
- Retention & destruction of documents as per Annexure-III.
- Following documents not be destroyed and has to be retained forever:
- BMR/BPR of batches which has been charged for stability.
- BMR/BPR of batches in which validation has been carried out.
- Stability data & reports, Vendor audit reports & Technology transfer document.
Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/
- DESTRUCTION OF DOCUMENTS AFTER COMPLETION OF RETENTION TIME:
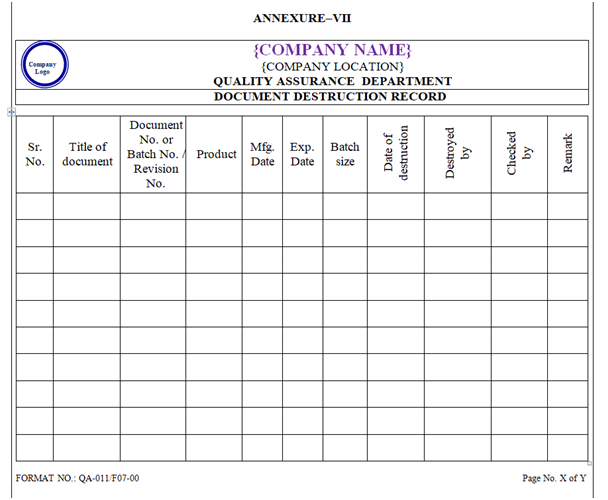
- The documents for which the retention time is over to be destroyed by shredding. Record of the destroyed documents to be maintained in the destruction record as per Annexure-VII.
- Destruction shall be carried out in presence of QA officer/executive.
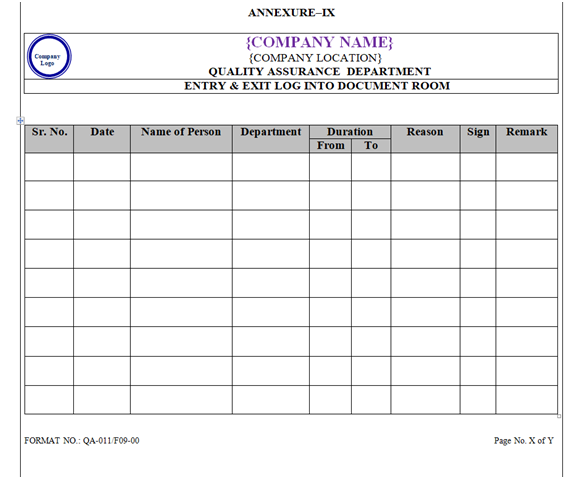
- Entry & Exit into QA Document Room: Only authorized persons shall be allowed to enter in document room and maintain the entry exit log for document room as per Annexure –IX.
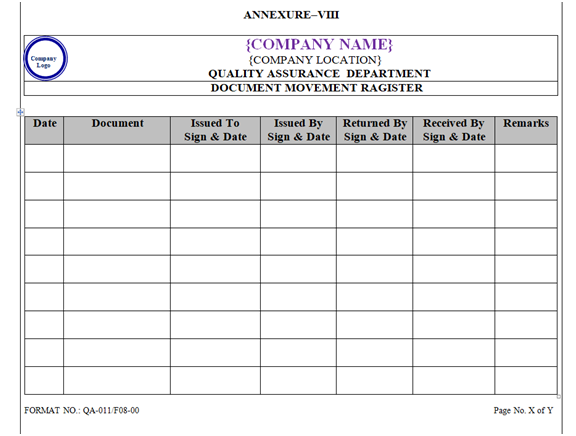
- Movement of Document: QA officer/executive shall maintain the document movement record as per Annexure-VIII.
- Photocopy of SOP, COA or any other document can be attached with incident/deviation, change control, OOS/OOT and other documents for reference purpose by putting red color stamp of “REFERENCE COPY” in footer in center of the document

- RETRIVAL / ARCHIVAL OF DOCUMENTS:
- QA Officer / Executive shall be responsible for retrieval of the previous version of documents before issuing revised copy. The distribution of the documents shall be carried out by QA only.
- All Executed Retrieved Documents shall be archived in QA Department with lock and key arrangements and the records shall be easily retrievable.
- Only the current version of document shall be available at the place of use
- All the retrieved issued document/ Log book/bound book shall be recorded in format as shown in Annexure-II.
- All completed documents of all departments shall be submitted to QA department on monthly basis.
- Bound books shall be submitted to QA Department after complete filling.
- DATA CONTROL:
- All the data maintained in software shall be protected by Password Assessable to QA Head / respective Head of the Department and to the second person in the Department assign for operation by Head of the Department.
- Any reprocessing in the data generated by software shall not be allowed without prior approval from QA Head.
- Any reprocessed data shall have audit trail.
- Archiving of data in External Hard Disc shall be done by IT and all such disc shall be kept under lock and key with Concerned Department. The keys shall be kept with the QA Head.
- The distribution of data to other than user shall be done only after approval of QA Head.
- Automatic Calculations and results made by the software shall be validated by the user on a defined frequency.
- REFERENCES:
US Code of Federal Regulations, Current Good Manufacturing Practice for Finished Pharmaceuticals (Part 211), Food and Drug Administration, DHHS, 21 CFR, CH.1, 4 -1- 95 Edition.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE | FORMAT No. |
| Annexure-I | Request Form for Issuance of Documents / Formats | QA-011/F01-00 |
| Annexure-II | Documents / Formats Issuance, Retrieval & Destruction Log | QA-011/F02-00 |
| Annexure-III | Retention & Destruction of Documents | QA-011/F03-00 |
| Annexure-IV | Issuance And Retrieval Form For Specifications, STP & GTP | QA-011/F04-00 |
| Annexure-V | Stamps Location Specimen | QA-011/F05-00 |
| Annexure-VI | Rack Index | QA-011/F06-00 |
| Annexure-VII | Document Destruction Record | QA-011/F07-00 |
| Annexure-VIII | Document Movement Register | QA-011/F08-00 |
| Annexure-IX | Entry & Exit Log into Document Room | QA-011/F09-00 |
| Annexure-X | Obsolete Document Record | QA-011/F10-00 |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Controlled Copy No. 03 : Head Warehouse
- Controlled Copy No. 04 : Head Production
- Controlled Copy No. 05 : Head Engineering
- Controlled Copy No. 06 : Human Resources
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| MFR | : | Master Formula Record |
| BMR | : | Batch Manufacturing Record |
| BPR | : | Batch Packing Record |
| Ltd. | : | Limited |
| QA | : | Quality Assurance |
| QC | : | Quality Control |
| S. No. | : | Serial Number |
| : | Portable Document Format | |
| HOD | : | Head of the Department |
| No. | : | Number |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be recorded manual |
Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/

Click the link and download the word file of this procedure: https://pharmaguidehub.com/product/documentation-and-data-control/