- OBJECTIVE:
To lay down a Procedure for Specimen Signatures.
- SCOPE:
This SOP is applicable to all employees of {Company Name} {Company Location}, who are involved in signing during Preparation, Review, Approval of Documents.
- RESPONSIBILITY:
- QA Executive / Designee shall be responsible to verify the specimen signature.
- Concerned department designee shall be responsible to maintain specimen signature log.
- ACCOUNTABILITY:
QA Head shall be accountable for implementation of this SOP.
- PROCEDURE:
- DEFINITION:
“Specimen Signature” is a Signature of an employee to proof authenticity of a person who is signing the documents. Specimen Signatures shall provide documentary evidence for signing the particular documents and performing particular jobs.
- Specimen Signature shall provide a traceability of the documents signed by that particular employee.
- Specimen Signature shall avoid data integrity of the documents.
- Specimen Signature of each & every employee, who is responsible for signing the documents, shall be maintained.
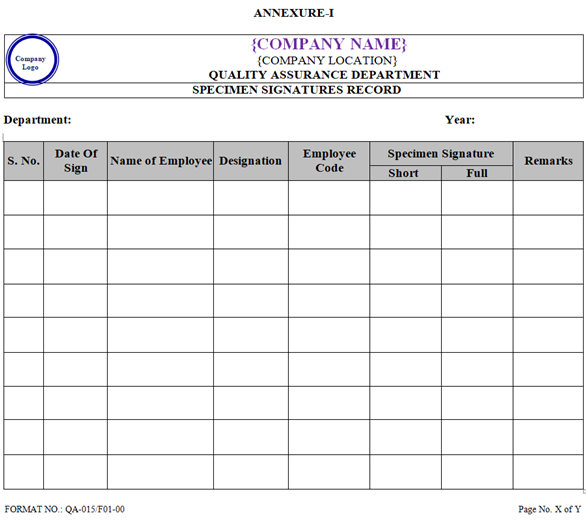
- Concerned Department Head or his / her designee shall maintain the record of Specimen Signatures as per format shown in Annexure-I.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/specimen-signatures/
- Original Record of Specimen Signature shall be submitted to QA Department and QA shall issue controlled Copy to all Departments.
- Employee shall sign in the record of Specimen Signatures after completion of Induction Training as per Annexure-I.
- Employee shall not change the Signatures during the signing the Documents. Same Signatures shall be used in all documents as per “Specimen Signatures Record”.
- When any employee resigns or transfers to other location HR Department shall informed to QA and record of Specimen Signatures shall be updated by QA in the remarks column by writing Resigned or transferred.
- Concerned Head / designee shall update the record on yearly basis or whenever required.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE | FORMAT No. |
| Annexure- I | Specimen Signatures Record | QA-015/F01-00 |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Controlled Copy No. 03 : Head Production
- Controlled Copy No. 04 : Head Engineering
- Controlled Copy No. 05 : Head Human Resource
- Controlled Copy No. 06 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| Ltd. | : | Limited |
| QA | : | Quality Assurance |
| HR | : | Human Resource |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Introduction of New SOP | To be written manual |
ANNEXURE-I
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/specimen-signatures/