- OBJECTIVE:
To lay down a procedure for Procurement, Use and Destruction of Stamps.
- SCOPE:
This SOP is applicable for Procurement, Use and Destruction of Stamps at {Company Name} {Company Location}.
- RESPONSIBILITY:
All Department personnel – Follow the instruction as per procedure.
QA Head –Training, approval & implementation of SOP.
- ACCOUNTABILITY:
QA Head shall be accountable for implementation and compliance of this SOP
- PROCEDURE:
- All stamps shall be procured by out sources through purchase department.
- User department officer/executive raises the indent & provide the matter of stamp to purchase department.
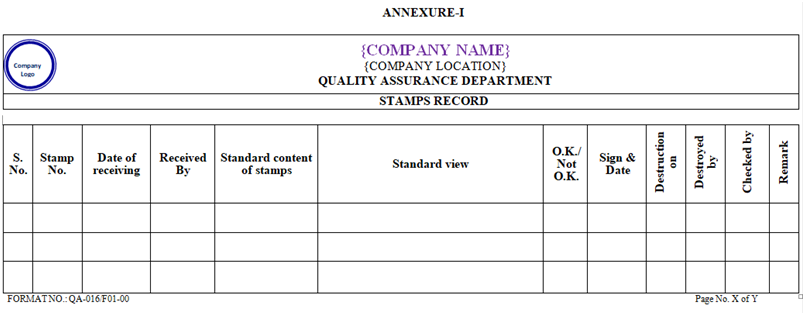
- After receiving of new stamps QA Officer/Executive shall check the matter as per Annexure-I.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/destruction-of-stamps/
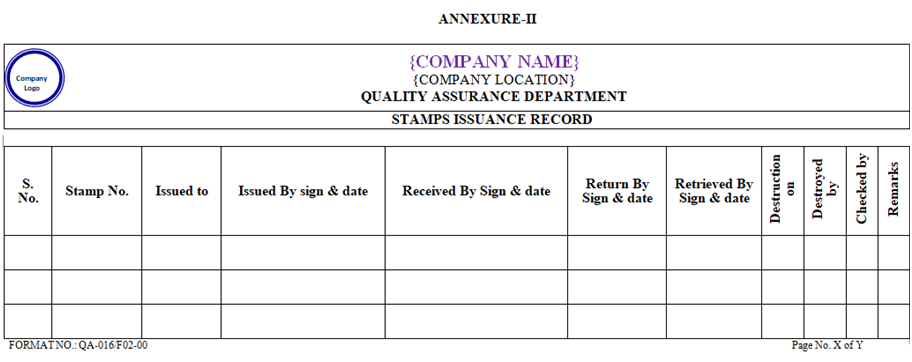
- All stamps shall be issued by QA as per ANNEXURE-II.
- All stamps shall be stored in the custody of concerned Department under lock & key.
- Stamps shall be used carefully & keep into the respective place after use.
- QA officer/executive shall destroy the stamps if found any defect/misprint.
- As per requirement similar stamp can be prepare more than one number.
- REFERENCES:
- Not Applicable
- ANNEXURES:
- ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Quality Control |
| Controlled Copy No. 03 | : | Head Production |
| Controlled Copy No. 04 | : | Head Warehouse |
| Master Copy | : | Quality Assurance Department |
ABBREVIATIONS:
| QA | : | Quality Assurance |
| SOP | : | Standard Operating Procedure |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE-I
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/destruction-of-stamps/
ANNEXURE – II
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/destruction-of-stamps/