OBJECTIVE:
To lay down the procedure for Environmental monitoring Program in manufacturing area and Microbiology area by using settle plate method (Passive air sampling) and air sampling method (Active air sampling).
SCOPE:
This SOP is applicable to the procedure Environmental monitoring Program in manufacturing area and Microbiology area at {Company Name} {Location}.
RESPONSIBILITY:
- Officer/Executive/Designee (QC) Micro. – Shall be responsible to follow the procedure as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
About Environment Monitoring:
Microbiological environmental monitoring (MEM) in pharmaceutical manufacturing is a critical process to ensure product sterility and safety. It involves assessing the microbial quality of controlled environments like cleanrooms and aseptic processing areas.
Various techniques are employed, including air sampling (e.g., settle plates, active air samplers), surface monitoring (e.g., contact plates, swabs), and personnel monitoring. The collected samples are then incubated and analyzed to identify and quantify the microorganisms present.
MEM data helps to evaluate the effectiveness of cleaning and disinfection procedures, identify potential sources of contamination, and maintain a controlled environment conducive to pharmaceutical production. Regular monitoring and trend analysis are essential to ensure ongoing compliance with regulatory standards and maintain product quality.
PROCEDURE:
Preparation of Environmental Monitoring Schedule:
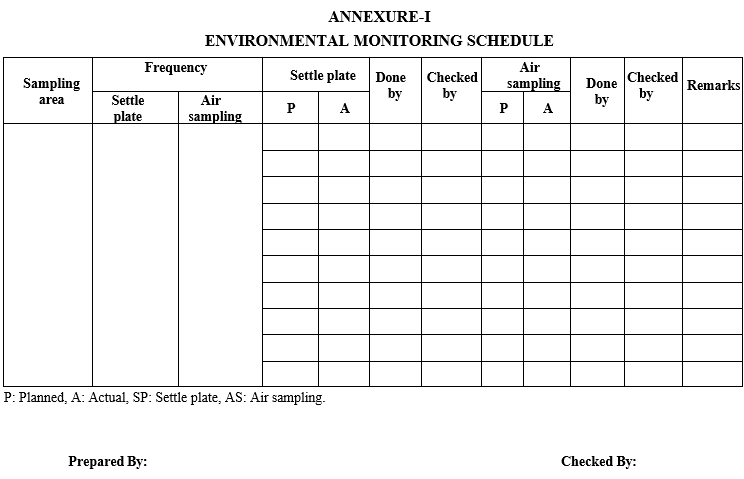
Environmental monitoring schedule shall be prepared for all the areas as per Format-I.
Preparation and transfer of monitoring accessories to manufacturing areas:
Prepare Soya bean casein digest agar and perform GPT as per SOP.
Pre incubate the Soyabean casein digest agar plates at 30-35°C for 24 – 48 hrs. and observe for any contamination. Discard the contaminated plates and update the same as per SOP.
Sanitize the surfaces of the SS sampling box with 70 % v/v IPA / Sterillium using a lint free cloth. Carry the pre-incubated SCD plates to the respective area in SS Sampling box.
Note: Follow the entry and exit procedure for the respective areas.
Environmental monitoring Method:
Settle Plate Method:
Refer the Sampling schedule (Format -I) and sampling Location layout Schematic diagram (Format -II).
Wear the hand gloves and disinfect with 70 % v/v IPA/Sterillium.
Take out the SCD plates from the SS Sampling box and label them with the following details.
- Name of the area
- Sampling Point Number
- A.R. Number
- Name of the Analyst
- Date of monitoring.
Remove the lid of the SCD plate, expose at each designated location for a period of 4 hours.
After completion of exposure close the plates and collect them in the SS sampling box and return to microbiology lab for incubation.
Laminar air flow units located in microbiology testing area shall be monitored in such a way that working activity shall be monitored on daily basis.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/environmental-monitoring-program-in-manufacturing-area-and-microbiology-area/
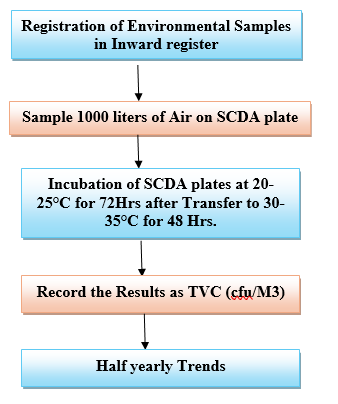
Active Air Sampling:
Refer to Sampling schedule (Format -I) and Sampling Location Schematic diagram (Format -II).
Wear the hand gloves and disinfect with the 70 % v/v IPA / Sterillium.
Label the SCD plates with the following details,
- Name of the area
- Sampling Point Number
- A.R. Number
- Name of the Analyst
- Date of monitoring.
Remove the lid of Petri plate and place the Petri plate in the Air sampler.
Place the air sampler in sampling location and carry out the Air sampling (1000 liters) as per SOP.
After completion of air sampling, carefully remove the plate from air sampler and close the lid.
After sampling, transfer the all plates in to the S.S Sampling box and return to microbiology laboratory for incubation.
Note: During environmental monitoring if spillage or breakage occurs, follow as per the SOP.
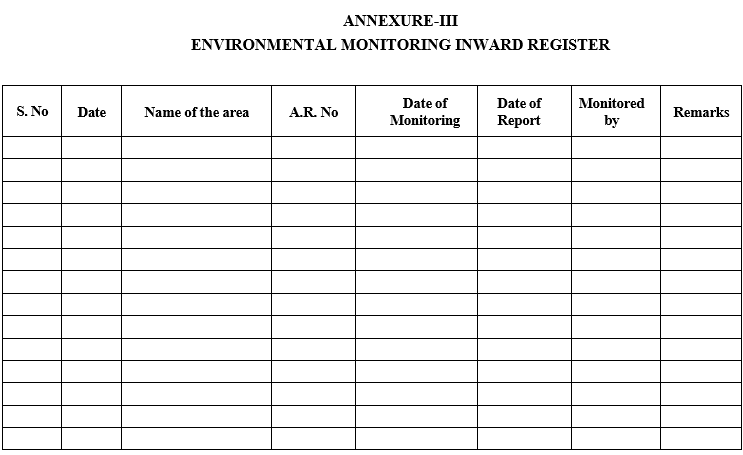
Registration of Environmental monitoring samples:
Samples shall be registered manually in inward register as per Format-III.
The manual A.R. No. shall have a unique numbering system.
Manual A.R. No. shall comprises of sixteen characters such as
EMR/MB/XX/0001/26
Where,
The First three characters “EMR” represents the Environmental Monitoring Report. The next character “/”indicates Slash.
The next character “MB” indicates department (Microbiology)
The next character “/”indicates Slash
The next Two characters “XX” represent the Monitoring Method. (SP-Incase of Settle plate, AS-Incase of Air sampling)
The next character “/”indicates slash.
The next four Digits “0001” indicates the serial number in the particular year.
The next character “/”indicates slash.
The next two digits “26”represents year 2026.
For E.g.: EMR/MB/SP/0001/26 indicates the first Environmental Monitoring report by Settle plate method in the Year of 2026
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/environmental-monitoring-program-in-manufacturing-area-and-microbiology-area/
Incubation of Environmental samples:
For Settle plate:
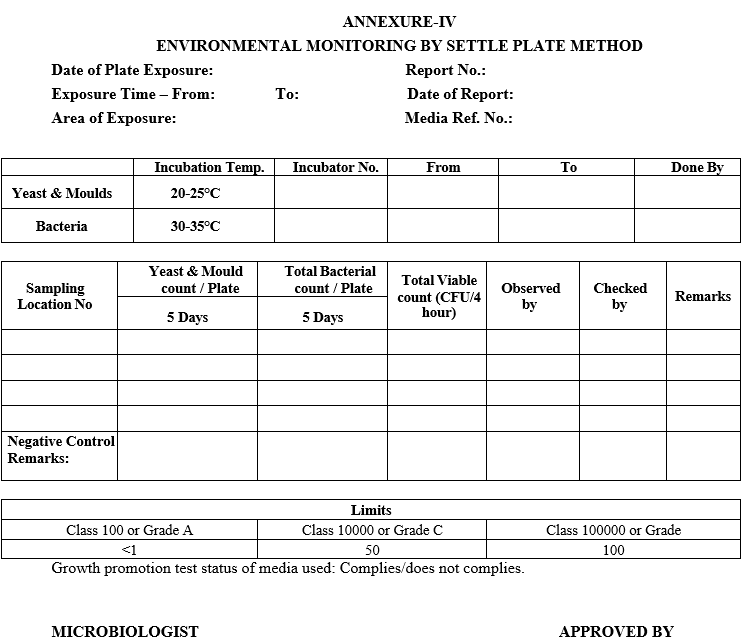
Incubate the exposed SCD plates in an inverted position at 20 to 25 0C for 72 hrs. and followed by 30°C to 35°C for 48 hrs. respectively.
Similarly incubate an unexposed SCD plate as a negative control.
After completion of 5 Days incubation period count the number of colony forming units on the SCD plate by using colony counter.
Negative control plate should not show any growth.
Read and record the results in (CFU/Plate) Format-IV.
Negative control plate should simulate the actual material movement process without exposure.
For Air Sampling:
Incubate the SCD plates in an inverted position in incubator at 20°C –25°C for 72 Hrs. and followed by 30 -35°C for 48 Hrs. respectively.
Similarly incubate an unexposed SCD plate as a negative control.
After completion of 5 Days incubation period count the number of colony forming units on the SCD plate by using colony counter.
Report the results as cfu /m3.
Negative control plate should not show any growth.
Record the results in Format-V.
Prior to release of the report, ensure the Growth promotion status is meeting the requirement.
Negative control plate should simulate the actual material movement process without exposure.
Monitoring Criteria on scheduled holidays:
If the area is kept idle, monitoring shall be stopped for that period. Monitoring shall be carried out after the resume of production activities as per schedule.
Whenever there is a holiday on scheduled day, monitoring shall be carried out on the next working day.
Acceptance Criteria For Routine Monitoring (In operation):
Settle Plate Method:
| CLASS | TOTAL VIABLE COUNT in cfu/4 hour |
| Specification Limit | |
| Class 100 (ISO class 5)/Grade A | < 1 |
| Grade A air supply* | 5** |
| Class 10,000 (ISO class 7)/ Grade C | 50 |
| Class 1,00,000 (ISO class 8)/Grade D | 100 |
Air Sampling Method:
| CLASS | TOTAL VIABLE COUNT in cfu/m3 |
| Specification Limit | |
| Class 100 (ISO class 5)/Grade A | < 1 |
| Grade A air supply* | 10** |
| Class 10,000 (ISO class 7)/ Grade C | 100 |
| Class 1,00,000 (ISO class 8)/Grade D | 200 |
* Area to be considered as “Grade A air supply”: LAF/RLAF with background Grade of C or Grade D used for sampling/dispensing and Dynamic Pass boxes with background of Grade C or Grade D.
** Grade A air supply limits shall not exceed grade B limits
Sampling Frequency:
| Sampling Area | Sampling Frequency | ||
| Settle Plate | Air sampling | ||
| Microbiology Lab | LAF cabinets( Class 100 / Grade A) | Daily | Daily |
| Microbiology testing areas( Class 10000 / Grade C) | Weekly | Fortnightly | |
| Production/Microbiology areas (Class 100000/Grade D) | Monthly | Quarterly | |
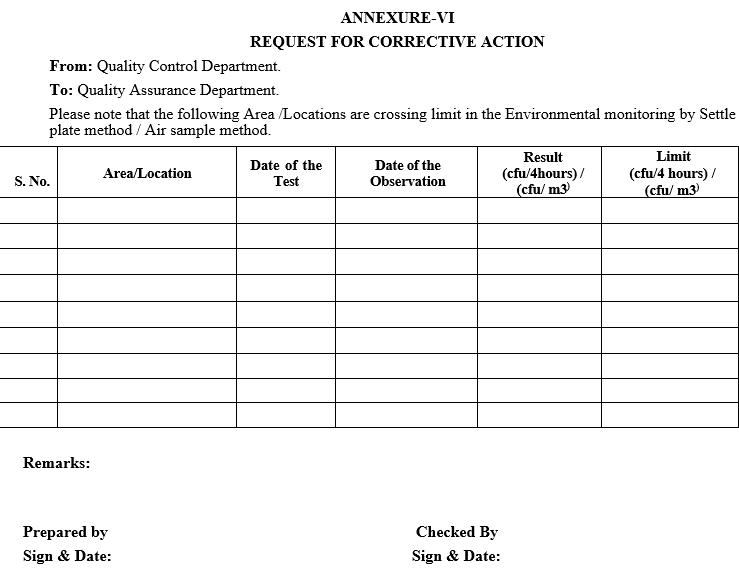
Handling of Out of Limit (OOL) results: If the Total viable count exceeds the limit, inform the Quality Assurance department as per Format-VI.
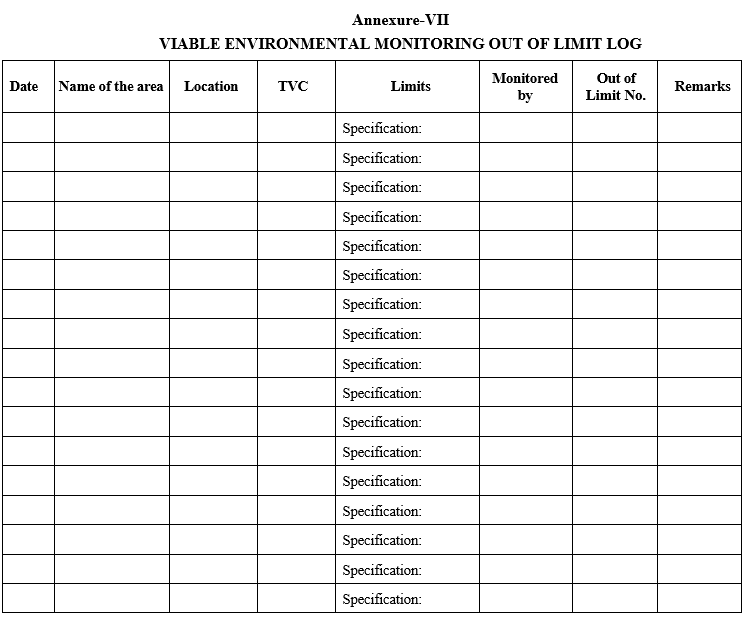
Assigning the Out of Limit (OOL) numbering system:
The details of the results shall be logged in Viable Environmental Monitoring out of Limit log as per Format – VII and the OOL Number shall be shall be allotted as
MB/OOL/YY/001,
where
MB – Microbiology
OOL – Out Of Limits
YY – Represent last two digit of current year
001 – Represent serial number starting with 001
Example: First OOL reported in year 2026 shall be numbered as MB/OOL/26/001
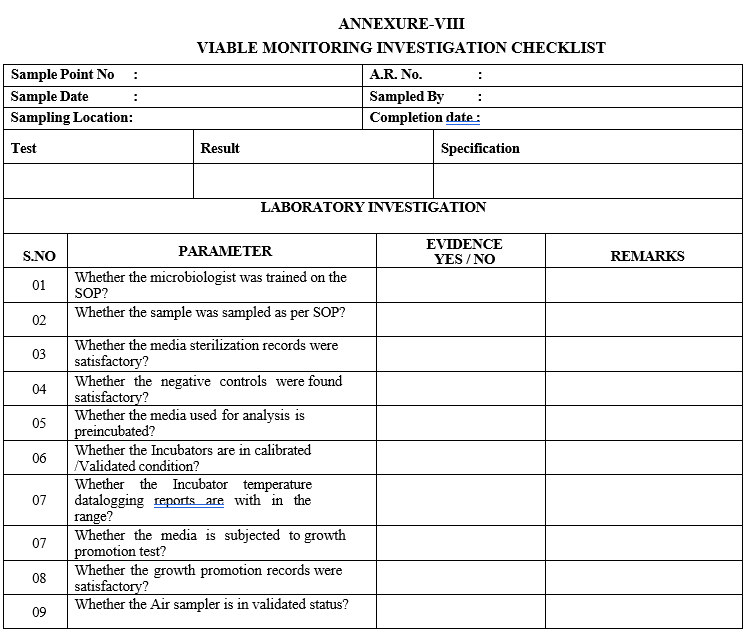
Initiate the laboratory investigation as per Format-VIII.
Based on the conclusion obtained after investigation, the corrective actions shall be taken.
Preparation of Trends:
Prepare a trend for each and every area in Microbiology testing area and LAF bench on monthly basis.
- Prepare a trend for each and every Sampling point in manufacturing areas by settle plate method on Quarterly basis.
Prepare a trend for each and every Sampling point in manufacturing areas by Air sampler method on Half yearly basis.
All trend shall prepared as per the Format -IX.
Assigning the drawing numbering system:
The drawing number shall have a unique numbering system.
Drawing number shall comprises of twelve characters such as
- DW/SL/001-00
- First two characters “DW” denote for drawings.
- Next character “/” indicate slash.
- Next two character “SL” denote for Sampling location Number”
- Next character “/” indicate slash.
- Next three characters “001” indicate serial number of the sampling location drawing.
- Next character “-“is a hyphen.
- The last two characters “00” is the revision number for drawing.
- After assigning the drawing numbers to sampling location drawings, it should be entered in the list of drawing of Format -X.
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Environmental monitoring schedule |
| Annexure-II | Schematic diagrams |
| Annexure-III | Environmental monitoring inward register |
| Annexure-IV | Report for Environmental Monitoring by Settle Plate Method |
| Annexure-V | Report for Environmental Monitoring by Air sampler Method |
| Annexure-VI | Request for corrective action |
| Annexure-VII | Viable environmental monitoring out of limit log |
| Annexure-VIII | Viable monitoring investigation checklist |
| Annexure-IX | Environmental Monitoring Trend Report |
| Annexure-X | List of Drawing Sampling Location |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head QC (Micro.)
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| cfu | : | Colony Forming Uni |
| SCD | : | Soyabean Casein Digest Agar. |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/environmental-monitoring-program-in-manufacturing-area-and-microbiology-area/
FLOW CHART

ANNEXURE-I
ENVIRONMENTAL MONITORING SCHEDULE
ANNEXURE-II
SCHEMATIC DIAGRAMS

ANNEXURE-III
ENVIRONMENTAL MONITORING INWARD REGISTER
ANNEXURE-IV
ENVIRONMENTAL MONITORING BY SETTLE PLATE METHOD
ANNEXURE-V
REPORT FOR ENVIRONMENTAL MONITORING BY AIR SAMPLER METHOD

ANNEXURE-VI
REQUEST FOR CORRECTIVE ACTION
Annexure-VII
VIABLE ENVIRONMENTAL MONITORING OUT OF LIMIT LOG
ANNEXURE-VIII
VIABLE MONITORING INVESTIGATION CHECKLIST
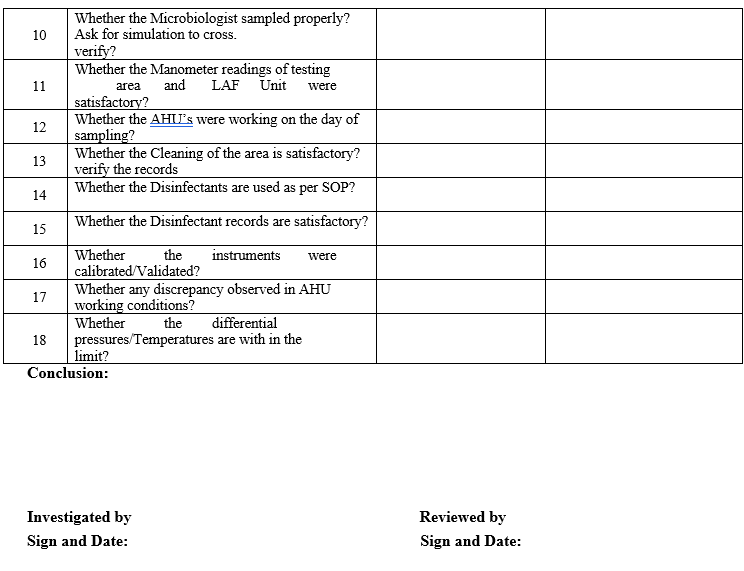
ANNEXURE-IX
ENVIRONMENTAL MONITORING TREND REPORT (SETTLE PALE /AIR SAMPLER METHOD)

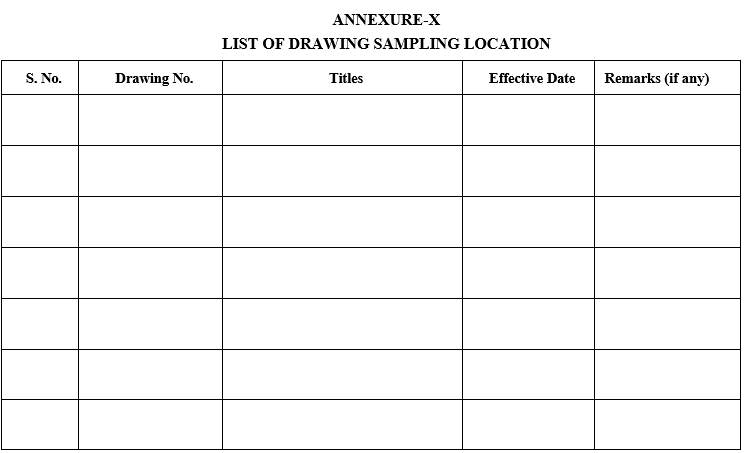
ANNEXURE-X
LIST OF DRAWING SAMPLING LOCATION
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/environmental-monitoring-program-in-manufacturing-area-and-microbiology-area/