OBJECTIVE:
To lay down the numbering system of sampling aids.
SCOPE:
This SOP is applicable to the numbering system of sampling aids at {Company Name} {Location}.
RESPONSIBILITY:
- QA Executive/designee shall be responsible to follow the procedures as per SOP.
- QA in charge shall be responsible for compliance of this SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
About Sampling Aids:
Sampling aids are essential tools in the pharmaceutical industry, used to collect representative samples of various substances for analysis and quality control. These aids ensure accurate and reliable results by minimizing contamination and ensuring proper sample collection techniques. Common sampling aids include scoops, spatulas, syringes, and specialized probes, each designed for specific types of materials like powders, liquids, or semi-solids. The choice of sampling aid depends on factors such as the nature of the material, the desired sample size, and the specific analytical requirements.
PROCEDURE:
Numbering of sampling aids (Unit dose sampler):
The numbering of Unit Dose Sampler consists of 8 characters
UDSBXXXX
UDS: represents the equipment code (UDS)
B: represents the Block
XXXX: represents the serial numerical number (E.g.: 0001, 0002, 0003 …. and so on) So, the first Unit Dose Sampler number shall be given as – UDSB0001
Numbering of Scoops and Spoons
The numbering of Scoop/Spoon consists of 8 characters
SPNBXXXX
SPN: represents the equipment code
B: represents the Block
XXXX: represent the serial numerical number (E.g.: 0001, 0002, 0003 ….and so on)
The first Scoop/Spoon number shall be given as – SPNB0001
Numbering of Sampling trays:
The numbering of Sampling tray consists of 8 characters
SATBXXXX
SAT: represents the equipment code
B: represents the Block
XXXX: represents the serial numerical number (E.g.: 0001, 0002, 0003 ….and so on)
The first Sampling tray number shall be given as – SATB0001
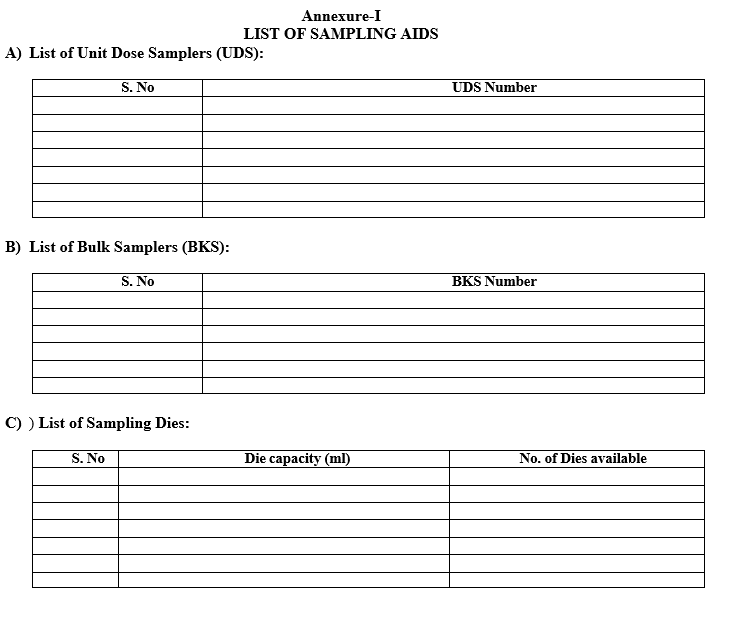
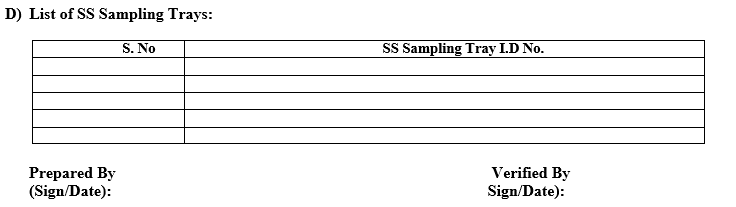
Refer the list of sampling Aids as per Annexure-I.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/numbering-of-sampling-aids/
Note:
After the sampling procedure the sampling aids shall be cleaned as per the SOP and such cleaned sampling aids are wrapped properly with stretch wrap film and stored.
Whenever new sampling aids will receive update the List of sampling Aids as per Annexure-I.
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | List of sampling Aids |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
Master Copy : Quality Assurance Department
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head Production
Controlled Copy No. 03 : Head Warehouse
ABBREVIATIONS:
| IPQA | : | In process quality assurance |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
LIST OF SAMPLING AIDS
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/numbering-of-sampling-aids/