OBJECTIVE:
To lay down the procedure for Sampling and Inspection of drug products by attributes in terms of Acceptance Quality Level (AQL) before release.
SCOPE:
This SOP is applicable to sampling and inspection of all bulk finish drug product before release for packing and pack finish drug product before release for distribution by attributes in terms of AQL at {Company Name}, {Location}.
RESPONSIBILITY:
Production Personnel:
To perform on-line sampling.
To intimate the Quality Assurance department for the inspection of batch samples.
To take appropriate action based upon the outcome of AQL inspection.
QA Personnel
To prepare and implement the SOP as per this guideline.
To do the AQL sampling and inspection as per SOP.
To take appropriate action based upon the outcome of AQL inspection.
Quality Assurance Manager:
To ensure that the AQL sampling inspection procedure is followed as per SOP.
To review and approve the investigation reports in case if batch does not meet AQL.
To ensure implementation the system.
ACCOUNTABILITY:
Head QA shall be accountable for approval and implementation of SOP.
PROCEDURE :
Sampling and inspection of bulk finish drug products:
Note: AQL sampling shall be performed at stages depends upon the mode of operation. i.e. either during on- line manufacturing or after the completion of visual inspection by Production department.
After completion of the manufacturing &/or inspection of drug product (i.e. after Compression or Coating of Tablets, after Capsule filling, production department shall intimate to the Quality Assurance department for AQL sampling and inspection of the batch.
QA Inspector shall perform the sampling & inspection in the same cubicle/area (i.e. Compression/ Filling/ Coating/ inspection room etc.). Before start of AQL sampling & inspection, QA Inspector shall check & ensure that environmental conditions are within the limit as mentioned in BMR and record the same in the AQL inspection report (Attachment-V).
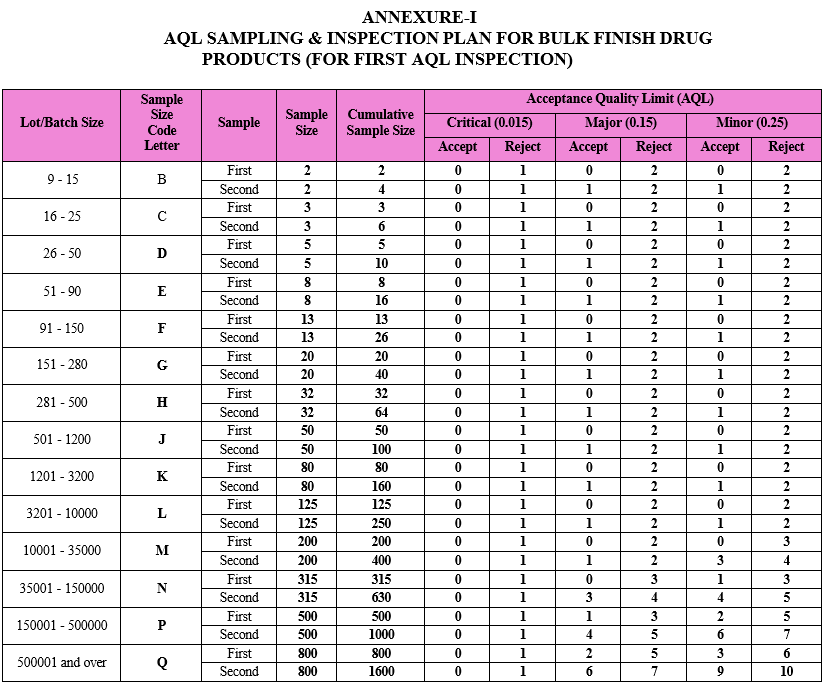
As per the batch size of the respective drug product, the sample size code letter and sample size shall be identified by QA from the AQL sampling inspection plan provided as Attachment-I. e.g. If batch size of the product is 100000 units, then for first AQL inspection, sample size code letter shall be N and first sample size shall be 315 units.
The number of samples first inspected shall be equal to the first sample size given in the sampling plan, i.e. Attachment-I.
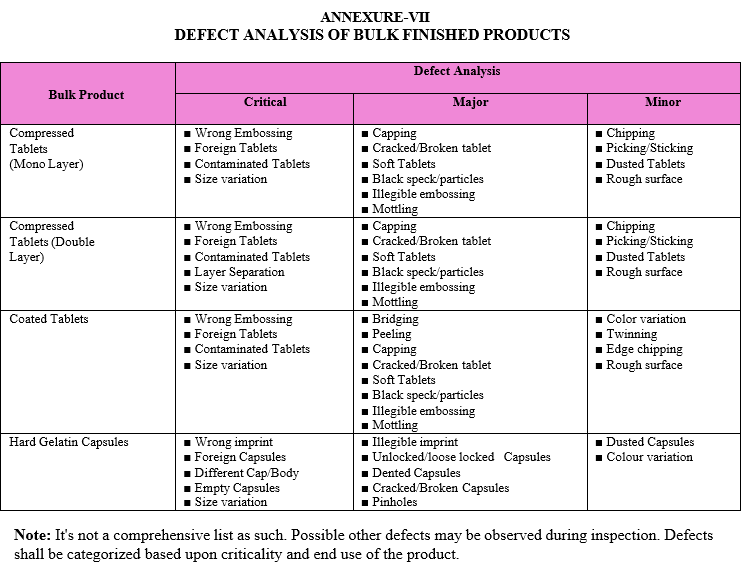
After collecting the sample, same shall be inspected for Critical, Major and Minor defects. Refer Attachment-VII for Critical, Major and Minor defects of various bulk finish drug products.
Compare the results of the first sample size inspection against the acceptance criteria as provided in Attachment-I.
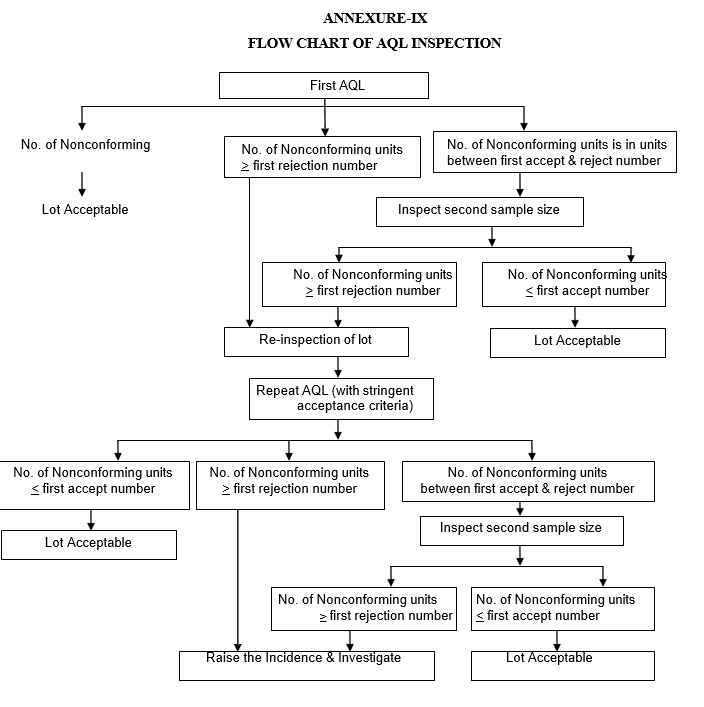
If the number of nonconforming items found in the first sample is equal to or less than the first acceptance number, the lot/batch shall be considered as acceptable.
e.g. For the batch size of 100000 units, 315 units (first sample size) shall be inspected for defects. If the number of nonconforming items found equal to or less than the first acceptance number, i.e. 0 for Critical & Major defects respectively & 1 for minor defects, the lot/batch shall be considered as acceptable.
If the number of nonconforming items found in the first sample is equal to or greater than the first rejection number, then lot shall be considered as not acceptable. In such case follow the procedure defined in the below paragraph.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/acceptance-quality-level/
e.g. In above example , if the number of nonconforming items found in the first sample (i.e. 315 units) is equal to or greater than the first rejection number, i.e. 1 for Critical defects & 3 for Major & Minor defects respectively, then lot shall be considered as not acceptable.
If the number of nonconforming items found in the first sample is between the first acceptance and rejection number, a second sample of the size given in the plan shall be inspected.
e.g. In above example of step , if the number of nonconforming items found in the first sample is between the first acceptance and rejection number, i.e. more than 0 for Critical defects, between 0 to 3 for Major defects and between 1 to 3 for Minor defects, then a second sample of the size given in the plan (i.e. 315 units) shall be inspected.
The number of nonconforming items found in the first and second samples shall be accumulated. If the cumulative number of nonconforming items is equal to or less than the second acceptance number, the lot shall be considered acceptable.
e.g. In above example of step, if the cumulative number of non-conforming items is equal to or less than the second acceptance number, i.e. 0 for Critical, 3 for Major and 4 for Minor defects respectively, then the lot shall be considered acceptable.
If the cumulative number of nonconforming items is equal to or greater than the second rejection number, the lot shall be considered as not acceptable. In such case follow the procedure defined in the below step.
e.g. In above example of step, if the cumulative number of nonconforming items is equal to or greater than the second rejection number, i.e. 1 for Critical, 4 for Major and 5 for Minor defects respectively, the lot shall be considered as not acceptable.
Record all the observations in the Attachment-V or in the provision provided in the respective BMR.
If the results observed within the acceptance criteria, mention the remark as “Satisfactory” with sign and date of inspector.
Attach the copy of filled “Bulk Finish Drug Product Inspection Report” (Attachment-V) with the respective Batch Manufacturing Record.
After completion of the inspection, destroy the defected samples as per SOP titled as “Destruction of materials or Products” and record the quantity in respective batch manufacturing record. Handover the inspected good samples to concern production supervisor, who shall add these samples in the last container of bulk product.
Handling of AQL failure of bulk finish drug products:
If the results are not as per acceptance criteria, mention the remark as “Not Satisfactory, 100 % re-inspection of the batch is to be carried out” along with sign and date of inspector in the Attachment-V or in the BMR, as applicable.
Responsible Production Supervisor shall conduct the re-inspection of the batch along with defect analysis (i.e. no. of tablets of each type of defect) and record the activities in the respective batch manufacturing record. Sampling of inspected bulk finish drug product shall be done by Production supervisor as per above step.
After the completion of re-inspection of the batch, Production Supervisor shall intimate to the QA inspector for repeat AQL sampling and inspection.
QA inspector shall perform the repeat AQL sampling and inspection as per below procedure.
As per the batch size of the respective drug product, the sample size code letter and sample size shall be identified by QA inspector from the AQL sampling inspection plan provided as Attachment-II.
The number of samples first inspected shall be equal to the first sample size given in the sampling plan, i.e. Attachment-II.
After collecting the sample, same shall be inspected for Critical, Major and Minor defects. Refer attachment- VII for Critical, Major and Minor defects of various bulk finish drug products.
Compare the results of the repeat AQL inspection against the acceptance criteria as provided in Attachment- II.
If the number of nonconforming items found in the first sample is equal to or less than the first acceptance number, the lot/batch shall be considered as acceptable.
If the number of nonconforming items found in the first sample is equal to or greater than the first rejection number, then lot shall be considered as not acceptable. Mention the remark as “Not Satisfactory” in the Attachment-V or in the BMR (as applicable) and intimate the same to concern Department Head, QA Head and Plant Head. Concern Department Head shall raise the deviation & carry out investigation as per SOP titled as “Incident Reporting and Investigation”. Root cause analysis for the failure shall be carried out as per SOP titled “Quality Risk Management” to find out the root cause and risk assessment shall be done simultaneously. CAPA shall be taken as per SOP titled “CAPA (Corrective and Preventive Action) handling procedure” based on the identified root cause or probable root cause.
If the number of nonconforming items found in the first sample is between the first acceptance and rejection number, a second sample of the size given in the plan shall be inspected.
The number of nonconforming items found in the first and second samples shall be accumulated. If the cumulative number of nonconforming items is equal to or less than the second acceptance number, the lot shall be considered acceptable.
If the cumulative number of nonconforming items is equal to or greater than the second rejection number, the lot shall be considered as not acceptable. Mention the remark as “Not Satisfactory” in the Attachment-V or in the BMR (as applicable) and intimate the same to concern Department Head, QA Head and Plant Head. Decision on further course of action or disposition of batch shall be taken by QA Head in consultation with Plant Head and CQA Head.
The samples along with the batch shall be stored in appropriate condition and with ‘Under Hold’ label as per Attachment- XII of SOP titled as “Status Labeling” by QA department until the completion of investigation and final decision on the lot.
After completion of the investigation, the defected sample shall be destroyed as per SOP titled as “Destruction of Materials or Products”.
If the decision is taken for destruction of the lot, it shall be destroyed as per SOP titled as “Destruction of Materials or Products”.
Sampling and inspection of pack finish drug products:
During Packing operation, packing department shall intimate to the Quality Assurance department for AQL sampling and inspection of the batch.
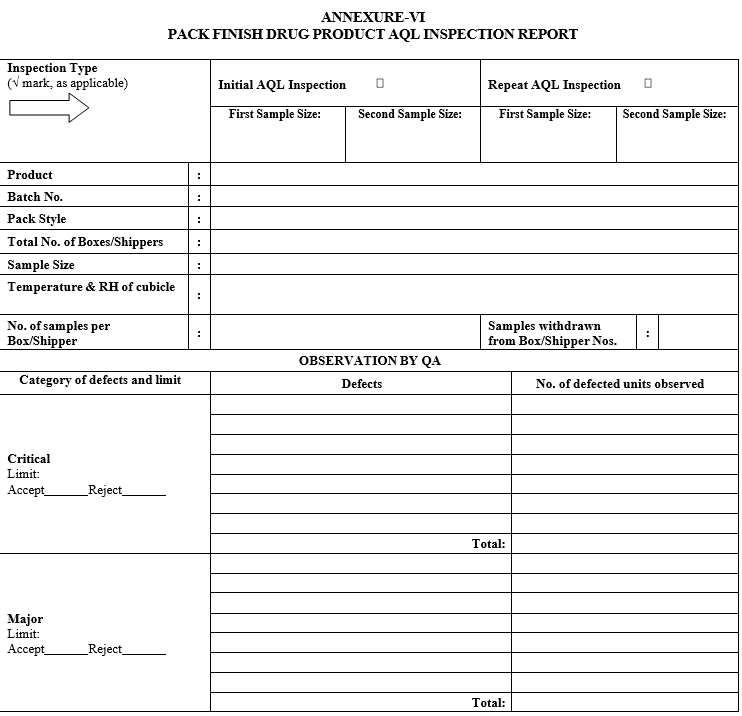
As per the batch size of the respective drug product, the sample size code letter and sample size shall be identified from the AQL sampling inspection plan provided in Attachment-III.
Note: Batch size of the pack finish drug product batch should be considered in terms of packed units as per respective pack style.
e.g.:
If the batch size of the Tablet drug product in manufacturing stage is 50000 Tablets and same is being packed in the 100 count bottle pack, then batch size at packing stage shall be 500 units (i.e. 500 bottles of 100 tablets each). In this case batch size as 500 units shall be considered for deriving sample size for AQL inspection. As per Attachment-III & IV, first sample size for this lot size shall be 32 units.
In above example, if batch after manufacturing is being packed in more than one pack style (e.g. 100 count bottle pack and 10 count blister pack), then batch size of individual pack style shall be considered based upon the quantity of bulk tablets assigned for each pack. For example, if 20000 tablets assigned for 100 count bottle pack and 30000 tablets assigned for 10 count blister pack, then batch size of bottle pack shall be 200 units and that of blister pack shall be 3000 units. Sample size for AQL inspection of individual pack style lot shall be derived based upon batch size of individual lot. In this case first sample size for AQL inspection shall be 20 units for bottle pack and 80 units for blister pack.
After getting the sample size, QA shall collect the representative samples uniformly from the pack line.
Note:
If total number of boxes/shippers of pack finish product is 10 or less than 10, then sample shall be collected from all the boxes/shippers.
If total number of boxes/shippers of pack finish product are more than 10, then calculate the value of √n + 1 (square root of n + 1).
Where “n” is the total number of pack finish product boxes/shippers.
If value of √n + 1 is less than 10, then sample shall be collected from at least 10 boxes/shippers. If value of √n + 1 is more than 10, then sample shall be collected from √n + 1 boxes/ shippers.
Example: If total number of sample is to be collected is 200 units and total number of boxes/shippers are 4, then 50 units shall be collected from initial, middle and end run of the packing operation considering time required to pack theoretical quantity.
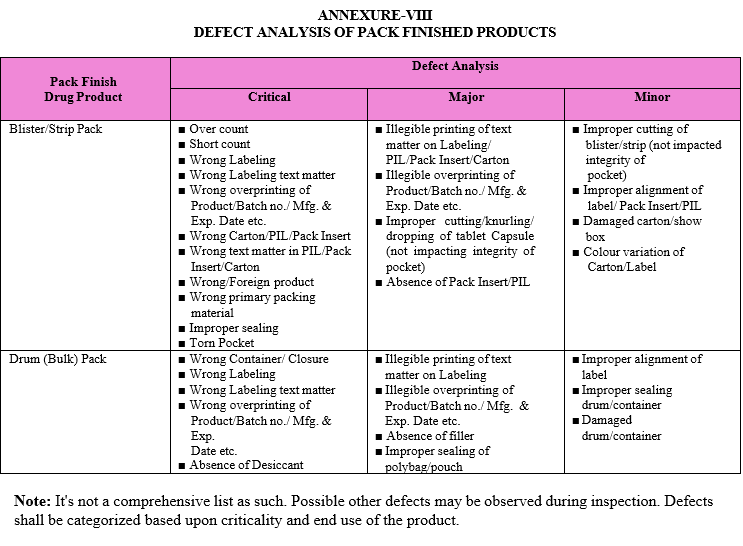
After collecting the sample, same shall be inspected for Critical, Major and Minor defects. Refer Attachment-VIII for Critical, Major and Minor defects of various pack finish drug products.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/acceptance-quality-level/
Compare the results of the first inspection against the acceptance criteria as provided in Attachment-III.
If the number of nonconforming items found in the first sample is equal to or less than the first acceptance number, the lot/batch shall be considered as acceptable.
If the number of nonconforming items found in the first sample is equal to or greater than the first rejection number, the lot shall be considered not acceptable. In such case follow the procedure defined in the following step.
If the number of nonconforming items found in the first sample is between the first acceptance and rejection number, a second sample of the size given in the plan shall be inspected
The number of nonconforming items found in the first and second samples shall be accumulated. If the cumulative number of nonconforming items is equal to or less than the second acceptance number, the lot shall be considered acceptable.
If the cumulative number of nonconforming items is equal to or greater than the second rejection number, the lot shall be considered as not acceptable. In such case follow the procedure defined in the below step no.
Record all the observations in the Attachment-VI or in the provision provided in the respective BPR.
If the results observed within the acceptance criteria, mention the remark as “Satisfactory” with sign and date of inspector.
Attach the copy of filled “Pack Finish Drug Product Inspection Report” (Attachment-VI) with the respective Batch Packing Record.
After completion of the inspection, handover the samples to packing officer or designee. Packing officer or designee shall bring the defected samples to the primary packing area/cubicle, open it, repack the inspected good bulk samples as per pack style and keep it in the respective boxes/shippers from which they are sampled as per standard procedure. If require, withdraw the samples from last box/shipper (loose shipper, if available) as the replacement sample (for defected samples destroyed) and repack them in the respective shippers. QA inspector shall ensure the proper repacking of good samples and destruction of defected samples. Record the activities in the “Pack Finish Drug Product Inspection Record” (Attachment-VI). Record the quantity of defected samples destroyed after AQL sampling inspection in the respective Batch Packing Record (BPR).
Handling of AQL failure of pack finish drug products:
If the results are not as per acceptance criteria, mention the remark as “Not Satisfactory, 100 % re-inspection of the batch is to be carried out” along with sign and date of inspector in the Attachment-VIII or BPR, as applicable.
Responsible Packing Supervisor shall conduct the re-inspection of the batch along with defect analysis (i.e. type of defects) and record the activities in the respective batch packing record.
QA inspector shall perform the repeat AQL sampling inspection as per below procedure.
As per the batch size of the respective drug product, the sample size code letter and sample size shall be identified by QA inspector from the AQL sampling inspection plan provided as Attachment-IV.
Note: Batch size of the pack finish drug product batch should be considered in terms of packed units as per respective pack style.
The number of samples first inspected shall be equal to the first sample size given in the sampling plan, i.e. Attachment-IV.
After collecting the sample, same shall be inspected for Critical, Major and Minor defects. Refer Attachment-VII for Critical, Major and Minor defects of various pack finish drug products.
Compare the results of the repeat AQL inspection against the acceptance criteria as provided in Attachment- IV.
If the number of nonconforming items found in the first sample is equal to or less than the first acceptance number, the lot/batch shall be considered as acceptable.
If the number of nonconforming items found in the first sample is equal to or greater than the first rejection number, then lot shall be considered as not acceptable. Mention the remark as “Not Satisfactory” in the Attachment-VI and intimate the same to concern Department Head, QA Head and Plant Head. Concern Department Head shall raise the deviation & carry out investigation as per SOP titled as “Incident Reporting and Investigation”. Root cause analysis for the failure shall be carried out as per SOP titled “Quality Risk Management” to find out the root cause and risk assessment shall be done simultaneously. CAPA shall be taken as per SOP “CAPA (Corrective and Preventive Action) handling procedure” based on the identified root cause or probable root cause.
If the number of nonconforming items found in the first sample is between the first acceptance and rejection number, a second sample of the size given in the plan shall be inspected.
The number of nonconforming items found in the first and second samples shall be accumulated. If the cumulative number of nonconforming items is equal to or less than the second acceptance number, the lot shall be considered acceptable.
If the cumulative number of nonconforming items is equal to or greater than the second rejection number, the lot shall be considered as not acceptable. Mention the remark as “Not Satisfactory” in the Attachment-VI and intimate the same to concern Department Head, QA Head and Plant Head. Decision on further course of action or disposition of batch shall be taken by QA Head in consultation with Plant Head and CQA Head.
The samples along with the batch shall be stored in appropriate condition with ‘Under Hold’ label as per Attachment- XII of SOP titled as “Status Labeling” by QA department until the completion of investigation and final decision on the batch.
After completion of the investigation, the defected sample shall be destroyed as per SOP titled as “Destruction of Materials or Products”.
If the decision is taken for destruction of the lot, it shall be destroyed as per SOP titled as “Destruction of Materials or Products”.
Definitions
Inspection by attributes: Inspection where by either the unit of product is classified simply as defective or no-defective, or the number of defects in the unit of product is counted, with respect to a given requirement or set of requirements.
Acceptable Quality Level (AQL): The Acceptance Quality Level (AQL) is defined as the maximum percent defective (or the maximum number of defects per hundred units) that, for purpose of sampling inspection, can be considered satisfactory as a process average.
Inspection Levels: The standard provides for three general inspection levels (i.e. Level I-Reduced Inspection, Level II- Normal Inspection, Level III- Tightened Inspection) and four special inspection levels. These levels permit the user to balance the cost of inspection against the amount of protection required. This guideline designed based on Normal Inspection level.
Normal Inspection: Normal inspection is that which is used where there is no evidence that the quality of product being submitted is better or poorer than the specified quality level.
Sampling Plan: A lot sampling plan is a statement of the sample size or sizes to be used and the associated acceptance and rejection numbers.
Representative Sampling: When appropriate, the number of units in the sample shall be selected in proportion to the size of sub-lots or sub-batches, or parts or the lot or batch, identified by some rationale criterion. When representative sampling is used, the units from each part of the lot or batch shall be selected at random.
Critical Defects: A critical defect is on that judgment and experience indicate is likely to result in hazardous or unsafe conditions for individuals using, maintaining, or depending upon the products.
Major Defects: A major defect is one, other than critical, that is likely to result in failure, or to reduce materially the usability of the unit of product for its intended purpose.
Minor Defects: A minor defect is one that is not likely to reduce materially the usability of the unit of product for its indented purpose, or is a departure from established standards having little bearing on the effective use or operation of the unit of product.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/acceptance-quality-level/
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | AQL sampling and inspection plan for bulk finish drug products (for first AQL inspection) |
| Annexure-II | AQL sampling and inspection plan for bulk finish drug products (for repeat AQL inspection) |
| Annexure-III | AQL sampling and inspection plan for pack finish drug products (for first AQL inspection) |
| Annexure-IV | AQL sampling and inspection plan for pack finish drug products (for repeat AQL inspection) |
| Annexure-V | Bulk finish drug product AQL inspection report |
| Annexure-VI | Pack finish drug product AQL inspection report |
| Annexure-VII | Defect analysis of bulk finished products |
| Annexure-VIII | Defect analysis of pack finished products |
| Annexure-IX | Flow chart of AQL inspection |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
| Controlled Copy No.01 | : | Manager Quality Assurance |
| Controlled Copy No.02 | : | Manager Production |
| Master Copy | : | Quality Assurance Department |
ABBREVIATIONS:
| SOP | : | Standard Operating Procedure |
| QA | : | Quality Assurance |
| AQL | : | Acceptance quality level |
| SOP | : | Standard operating procedure |
| etc. | : | Etcetera |
| i.e. | : | That is |
| e.g. | : | Example gratia |
| BMR | : | Batch manufacturing record |
| BPR | : | Batch packing record |
| QA | : | Quality Assurance |
| % | : | Percent |
| No. | : | Number |
| RH | : | Relative humidity |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be recorded manual |
ANNEXURE-I
AQL SAMPLING & INSPECTION PLAN FOR BULK FINISH DRUG PRODUCTS (FOR FIRST AQL INSPECTION)
ANNEXURE-II
AQL SAMPLING & INSPECTION PLAN FOR BULK FINISH DRUG PRODUCTS (FOR REPEAT AQL INSPECTION)
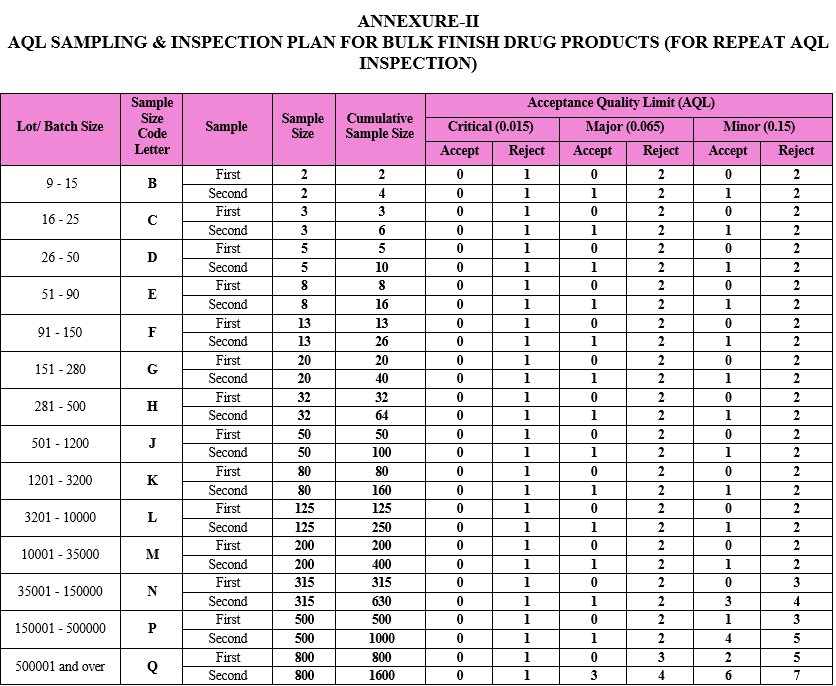
ANNEXURE-III
AQL SAMPLING & INSPECTION PLAN FOR PACK FINISH DRUG PRODUCTS (FOR FIRST AQL INSPECTION)
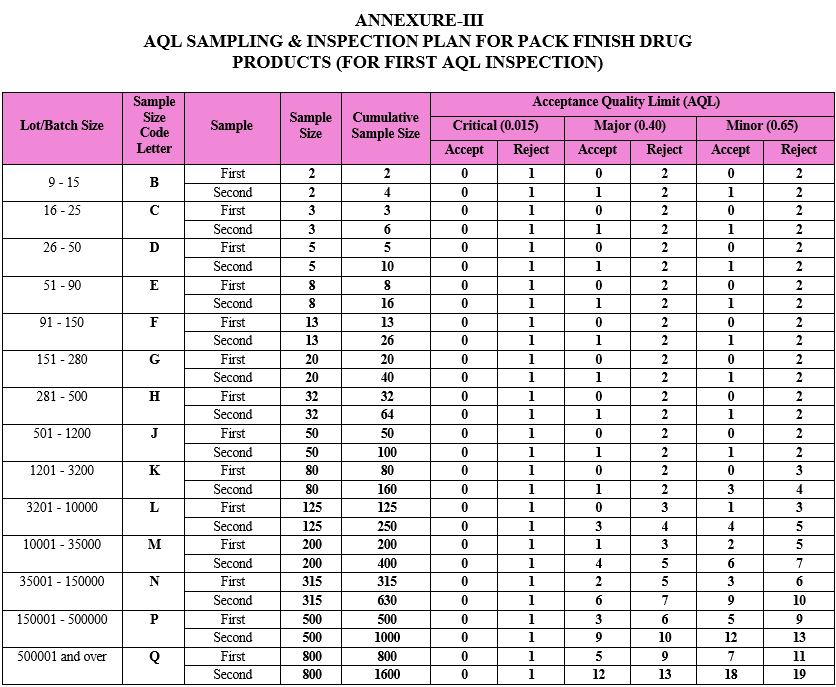
ANNEXURE-IV
AQL SAMPLING & INSPECTION PLAN FOR PACK FINISH DRUG PRODUCTS (FOR REPEAT AQL INSPECTION)
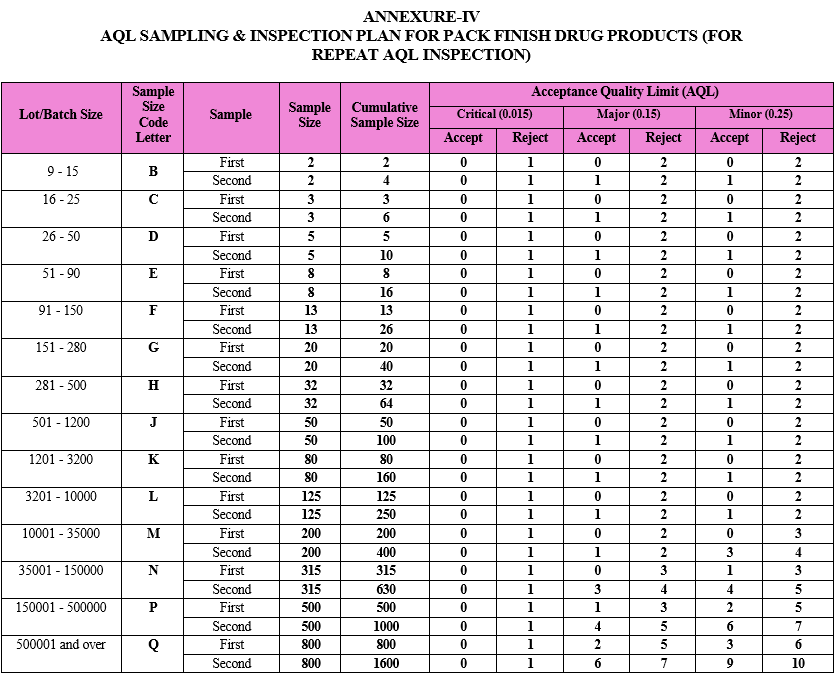
ANNEXURE-V
BULK FINISH DRUG PRODUCT AQL INSPECTION REPORT

Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/acceptance-quality-level/
ANNEXURE-VI
PACK FINISH DRUG PRODUCT AQL INSPECTION REPORT
ANNEXURE-VII
DEFECT ANALYSIS OF BULK FINISHED PRODUCTS
ANNEXURE-VIII
DEFECT ANALYSIS OF PACK FINISHED PRODUCTS
ANNEXURE-IX
FLOW CHART OF AQL INSPECTION
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/acceptance-quality-level/




