OBJECTIVE:
To lay down the procedure for Quarantining of In-process materials i.e.: blend /granules, tablets, filled capsules.
SCOPE:
This SOP is applicable to the procedure for Quarantining of In-process materials i.e.: blend /granules, tablets, filled capsules.
RESPONSIBILITY:
Initiator Officer/Designee: Production shall perform the operation activity as per SOP.
Initiator Executive/Designee: Production shall ensure the compliance of the SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
ABOUT QUARANTINING OF INPROCESS MATERIAL:
Quarantining material in pharmaceuticals is a critical process to ensure product safety and quality. It involves isolating incoming raw materials, packaging components, or finished products until they are thoroughly tested and approved for use. This prevents the inadvertent use of potentially contaminated or substandard materials in the manufacturing process.
Quarantine areas are clearly marked and access is restricted to authorized personnel. Materials are meticulously labeled with relevant information, including batch numbers, receipt dates, and quarantine status. Samples are taken for testing according to established procedures, and the quarantined materials are only released for use once the test results meet pre-defined specifications.
This practice is essential for complying with regulatory requirements, maintaining the integrity of the supply chain, and safeguarding public health by preventing the release of potentially harmful products.
PROCEDURE:
Collect the Blend/filled capsule/tablet in pre-labelled IPC / HDPE containers with double self sealing polybag and record the sample weights in BPRR and label.
Tightly close the both polybags neatly.
Store the blend in the blender bins if applicable.
Weigh the Blender bins and label accordingly.
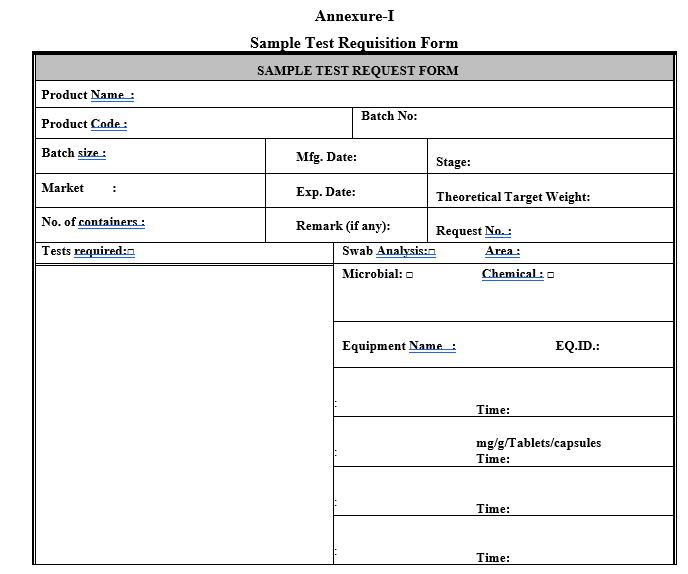
Intimate the Quality assurance to collect the sample for analysis through “Test Request Form” as per format-1, where ever required.
IPQA shall affix the “UNDER TEST” label on the container after sampling as per the SOP Sampling of Semi Finished and Finished Products.
Clean the outer surface of IPC /HDPE /Blender bins with lint free cloth and transfer the same to the blend hold area as per SOP
IPQA shall provide the “APPROVED / REJECTED” label as per results of analysis. These labels shall be provided along with “COA” and same label shall be pasted in BPRR.
IPQA shall provide the “APPROVED” label and affix the same in the respected bin/ HDPE crates/HDPE Containers as per SOP. Manufacturing Assurance personnel shall complete the review of the BPRR and In-process COA and the BPRR shall be signed off to release the batch for further processing, unless specified in respective blend BPRR as “Release the Blend / bulk for compression / filling along with parallel QC in process testing”.
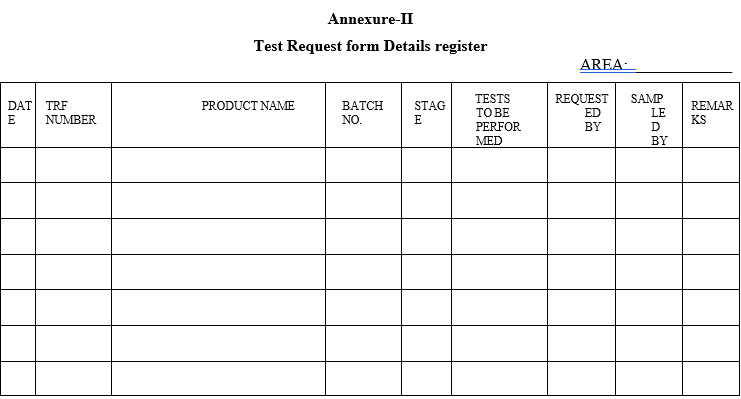
If the product is due for retesting, transfer the same product containers to the dedicated area and open the container in presence of QA for sampling against test requisition, enter the details in “Test request Form Details Register” as per format 1, kept in WIP hold area.
The same test request form as per format-1 can also be used for sending Swab Samples.
Product waiting for packing shall bear product label only in HDPE crates/ Containers.
The concerned executive has to fill all the relevant fields in “Test request form details register” as per format-2. ‘Requested by’ column shall be signed by concerned production executive and ‘Sampled by’ column shall be signed by concerned IPQA executive. Incase of swab samples ‘Sampled by’ column shall be signed by concerned QC executive.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quarantining-of-in-process-materials/
REFERENCES:
Not Applicable
ANNEXURES:
| Annexure Number | Title of annexure |
| Annexure-I | Sample Test Requisition Form |
| Annexure-II | Test Request form Details register |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
Master Copy : Quality Assurance Department
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head Production
ABBREVIATIONS:
| PD | : | Production |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| BMR | : | Batch manufacturing record |
| HDPE | : | High density polyethylene |
| IPQA | : | In process Quality Assurance |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Sample Test Requisition Form
Annexure-II
Test Request form Details register
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/quarantining-of-in-process-materials/




