- OBJECTIVE:
To lay down a Procedure for Qualification of Contract Analytical Laboratory.
- SCOPE:
This SOP is applicable for Assessment of outside Analytical Laboratory for Qualification and contract for analysis of samples sent by {Company Name} {Company Location}.
- RESPONSIBILITY:
Head QC
- ACCOUNTABILITY:
Head QA
PROCEDURE
Contract giver organization shall request a quality audit of the contract laboratory by the In charge- QA.
The contract laboratory audit must be performed by the In charge-Quality Control and In charge-QA or their designee to ensure compliance of laboratory as per cGMP/GLP (Refer Format-1).
After audit approval of contract laboratory by Contract giver, a written technical agreement shall be prepared as per the Format-2 which outlines confidentiality and responsibilities of both Contract giver and Contract laboratory.
STPs and specifications shall be sent to contract laboratory.
The contract laboratory shall be audited by Contract giver once in every 3 years.
However any major changes in the facility, terms and conditions (as per Contract giver organization), the contract laboratory shall be audited by Contract giver organization.
List of products and tests shall be updated laboratory wise as per “List of the Product / Materials” (as per Format-3) for every six months i.e. in the month of June and December and shall also be updated whenever there is an addition of products for testing.
List of the qualified contract laboratories shall be maintained by QA with details of Name of the laboratory, date of audit and next audit due date.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/qualification-of-contract-analytical-laboratory/
If any qualified contract laboratory receives warning letter from FDA then the contract laboratory shall be consider as disqualified for analysis.
The samples shall be sent along with test requisition form to the contract laboratory as per SOP
The contract laboratory shall perform the tests as per the procedures given by Contract giver organization.
The contract laboratory must use pharmacopoeial reference standards / working standards, standardized against relevant reference standard for analytical testing.
Any deviation from the STPs, prior intimation and concurrence to be obtained from Head- Quality Control of Contract giver organization.
Out of specification results of testing must be communicated to Head- Quality Control of Contract giver organization and the OOS investigation shall be performed as per the Contract giver organization SOP.
The contract laboratory is responsible for the review of raw data. Final COA / report shall be shared to Contract giver organization.
The Head- Quality Control of Contract giver organization or his designee shall be responsible for checking the final report of contract laboratory against the specifications.
After complete review of report, Head-Quality Control of Contract giver organization or his designee shall sign along with date on the final report of the contract laboratory.
The Head- Quality Control of Contract giver organization shall be responsible for the release/ reject of the products based on the final COA / report of the contract laboratory.
Technical agreement shall be revised only in case of change in the regulatory requirement / changes in the term & conditions of contract giver/contract accepter.
REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE | FORMAT No. |
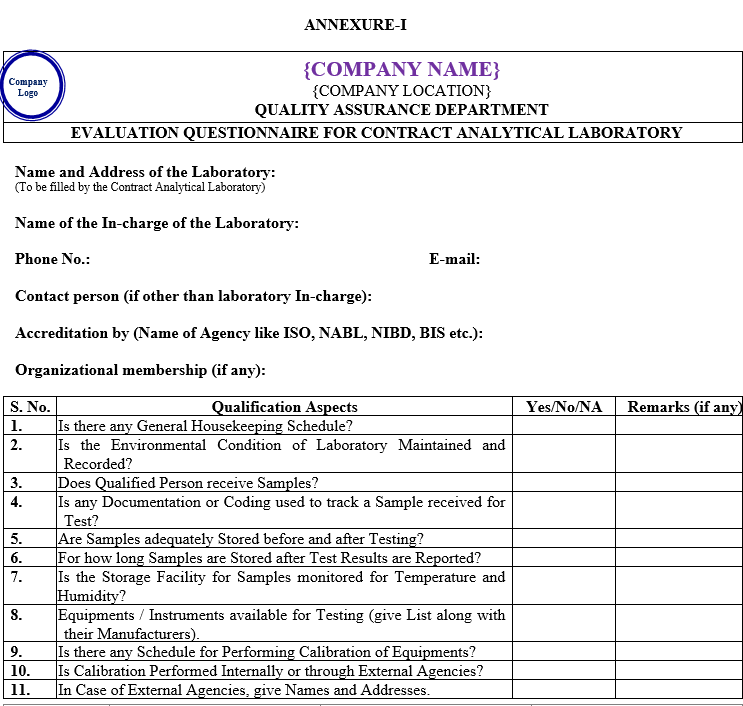
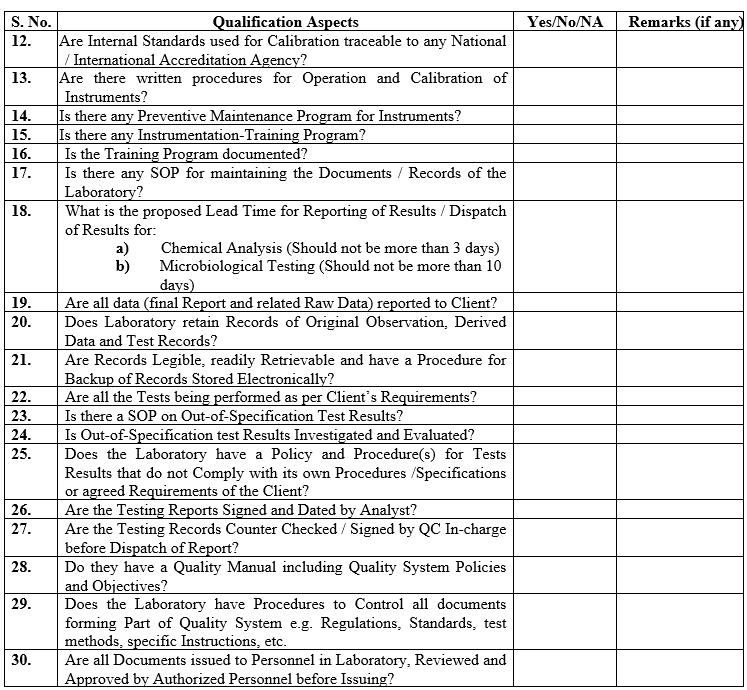
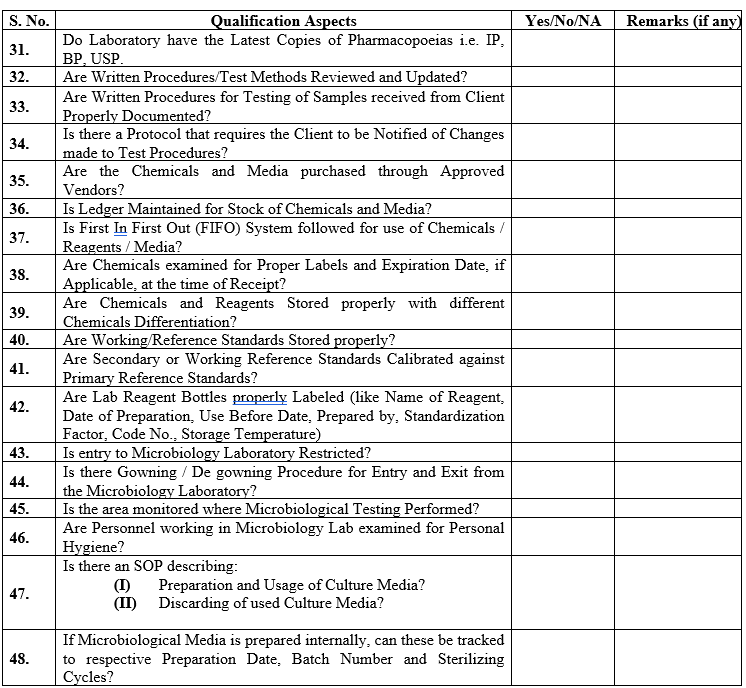
| Annexure-I | Evaluation Questionnaire For Contract Analytical Laboratory | QA-032/F01-00 |
| Annexure-II | Contract Analytical Laboratory Audit Log | QA-032/F02-00 |
| Annexure-III | Specimen for the Contract of Contract Analytical Laboratory | QA-032/F03-00 |
| Annexure-IV | Certificate-Qualification of Contract Analytical Laboratory | QA-032/F04-00 |
DISTRIBUTION:
| Controlled Copy No. 01 | : | Head Quality Assurance |
| Controlled Copy No. 02 | : | Head Quality Control |
| Master Copy | : | Quality Assurance Department |
ABBREVIATIONS:
| Ltd. | : | Limited |
| SOP | : | Standard Operating Procedure |
| No. | : | Number |
| QA | : | Quality Assurance |
| QC | : | Quality Control |
| QA | : | Quality Assurance |
| COA | : | Certificate of Analysis |
| LAF | : | Laminar Air Flow |
| STP | : | Standard Test Procedure |
| GLP | : | Good Laboratory Practices |
| HPLC | : | High Performance Liquid |
| UV | : | Ultraviolet |
| IP | : | Indian Pharmacopoeia |
| BP | : | British Pharmacopoeia |
| USP | : | United State Pharmacopoeia |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/qualification-of-contract-analytical-laboratory/
ANNEXURE-I
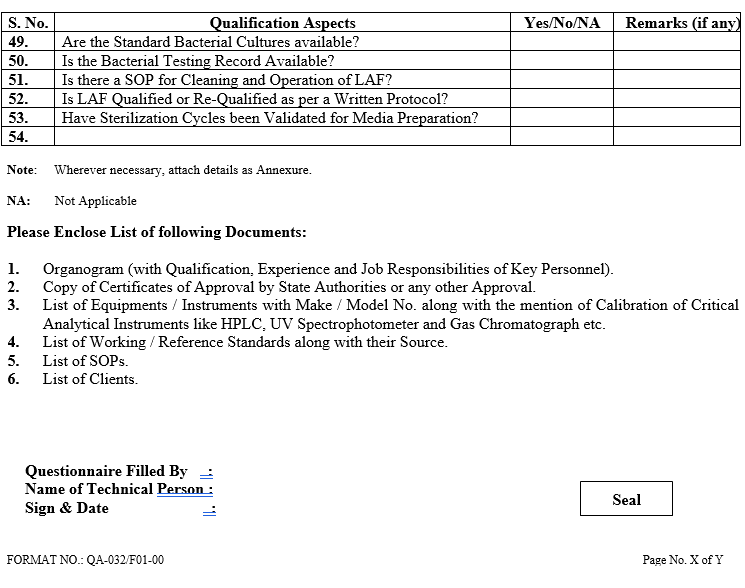
ANNEXURE- II
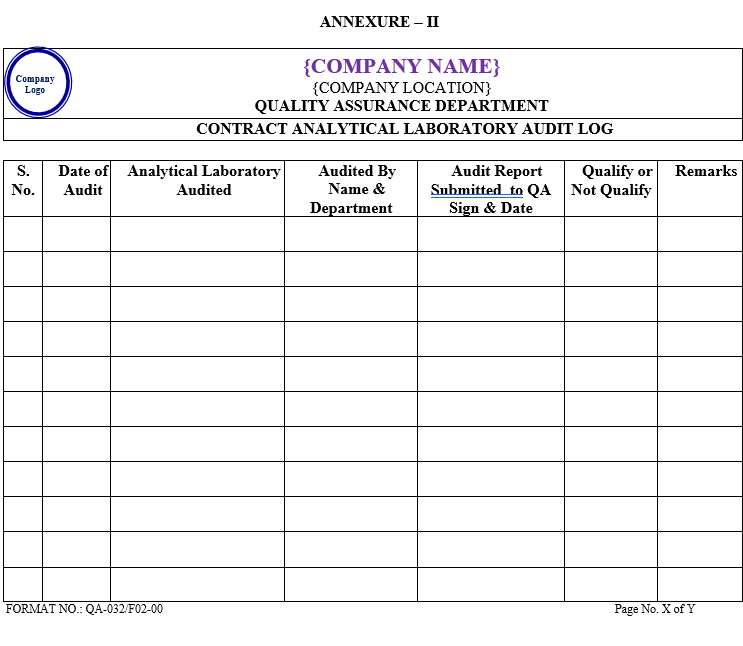
ANNEXURE – III


ANNEXURE – IV
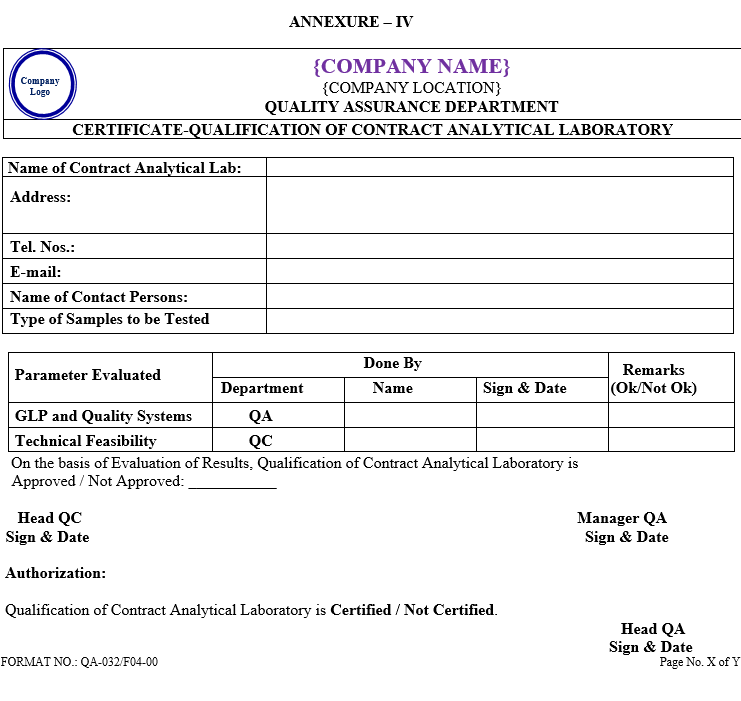
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/qualification-of-contract-analytical-laboratory/
Frequently Asked Question ?
- What qualifications should a contract analytical laboratory have to operate in the pharmaceutical industry?
- A contract analytical laboratory in the pharmaceutical industry should possess relevant accreditations and certifications, such as ISO 17025, demonstrating compliance with international standards for testing and calibration.
- Why is Good Laboratory Practice (GLP) essential for a contract analytical laboratory in the pharmaceutical sector?
- GLP ensures the reliability and integrity of data generated by the laboratory. It is crucial for maintaining quality and consistency in analytical testing, which is paramount in pharmaceutical research and development.
- What role does the expertise of laboratory personnel play in the qualification of a contract analytical laboratory?
- The qualifications and experience of laboratory personnel are critical factors. Trained and skilled professionals contribute to accurate and reliable analytical results, impacting the overall quality of pharmaceutical products.
- How does equipment qualification contribute to the credibility of a contract analytical laboratory?
- Proper qualification and calibration of analytical instruments are essential to ensure the accuracy and precision of test results. This contributes to the reliability of data generated by the laboratory.
- Why is it important for a contract analytical laboratory to establish and follow Standard Operating Procedures (SOPs)?
- SOPs provide a systematic and standardized approach to analytical testing. Following established procedures ensures consistency, traceability, and compliance with regulatory requirements.
- What is the significance of compliance with current Good Manufacturing Practices (cGMP) in a contract analytical laboratory?
- Adherence to cGMP is crucial to ensure that laboratory activities meet the quality standards required for pharmaceutical manufacturing. It includes maintaining a clean and controlled environment, proper documentation, and quality assurance.
- How does a contract analytical laboratory demonstrate data integrity in its operations?
- Implementing measures such as electronic data systems, audit trails, and data security protocols helps ensure the integrity of analytical data. This is vital for regulatory compliance and maintaining the credibility of the laboratory.
- What role does documentation play in the qualification of a contract analytical laboratory?
- Thorough documentation of procedures, methods, and results is essential for transparency and traceability. Accurate record-keeping supports the reproducibility of tests and aids in audits and regulatory inspections.
- How does a laboratory’s facility and environmental controls contribute to its qualification in the pharmaceutical industry?
- Adequate facilities, including controlled environmental conditions (temperature, humidity, cleanliness), are essential for maintaining the stability of samples and the accuracy of analytical testing. Proper facility design is critical to preventing contamination.
- Why is ongoing training and continuous improvement important for a contract analytical laboratory?
- Ongoing training ensures that laboratory personnel stay updated with the latest technologies, methods, and regulatory requirements. Continuous improvement initiatives help enhance efficiency, accuracy, and overall quality in laboratory operations.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/qualification-of-contract-analytical-laboratory/