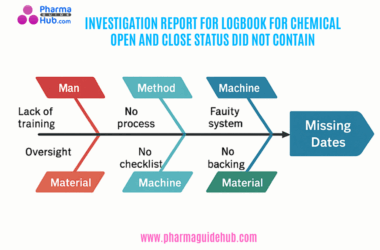
- OBJECTIVE:
To lay down a procedure for Annual Product Quality Review.
- SCOPE:
This SOP is applicable for the preparation of annual product quality review of all products to evaluate the consistency of the existing process at {Company Name} {Company Location}.
- RESPONSIBILITY:
- Designated QA person
- To prepare APQR.
- To collect and compile data for evaluation.
- Manager Production his /her Designee
- To review APQR
- To suggest CAPA/recommendation on the basis of APQR.
- Manager QA
- To review APQR.
- To suggest CAPA/recommendation on the basis of APQR.
- To ensure compilation & analysis.
- To ensure the APQR is performed as per timelines.
- To review suggested CAPA and to approve APQR reports.
- ACCOUNTABILITY:
Head QA
- PROCEDURE:
- APQR/IPQR Timelines:
- APQR shall be prepared for the product for which more than five batches are manufactured during the Calendar Year (Company Name).
- APQR shall be done for 12 months i.e. Jan. to Dec. of the calendar year.
- APQR shall be completed within 90 days. For example, APQR for products manufactured during the period of January to December 2015 shall be completed by Mar.2016.
- Schedule for Annual Product Quality review shall be Prepared.
- IPQR shall be prepared for every 3-month period in the first year of manufacturing as per the Annexure-I.
- APQR Numbering System
APQR shall be numbered as APQR/ABC/ZZ/NNN
Where,
APQR stands for Annual Product Quality Review
ABC stands for Product code.
ZZ stands for Year.
NNN stands for Serial No Start from 001,002,003………………
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/annual-product-quality-review/
- Annual Product Quality Review
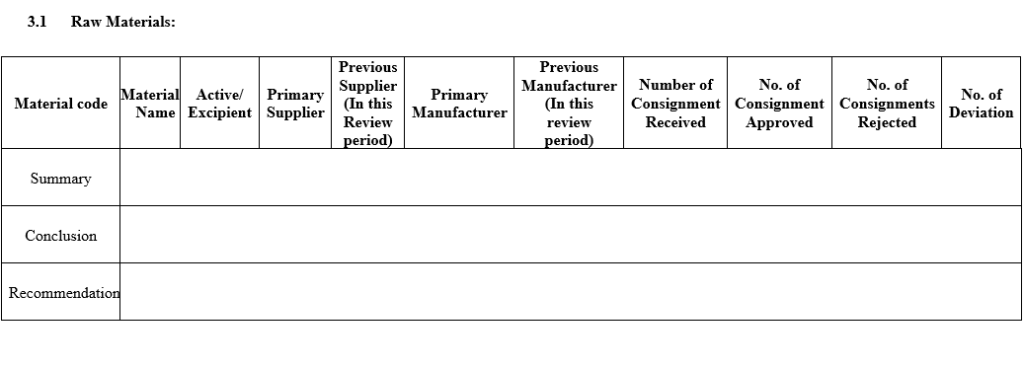
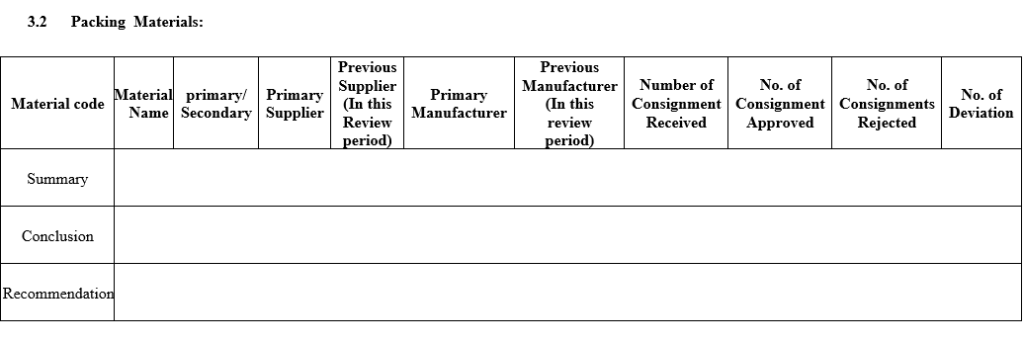
- The following parameters shall be reviewed during annual or periodic product Quality review for any product and report should be prepared as per Annexure-II.
- APQR shall be grouped for similar products (Formulation, Scientifically, justified, primary packing same and different brand name).
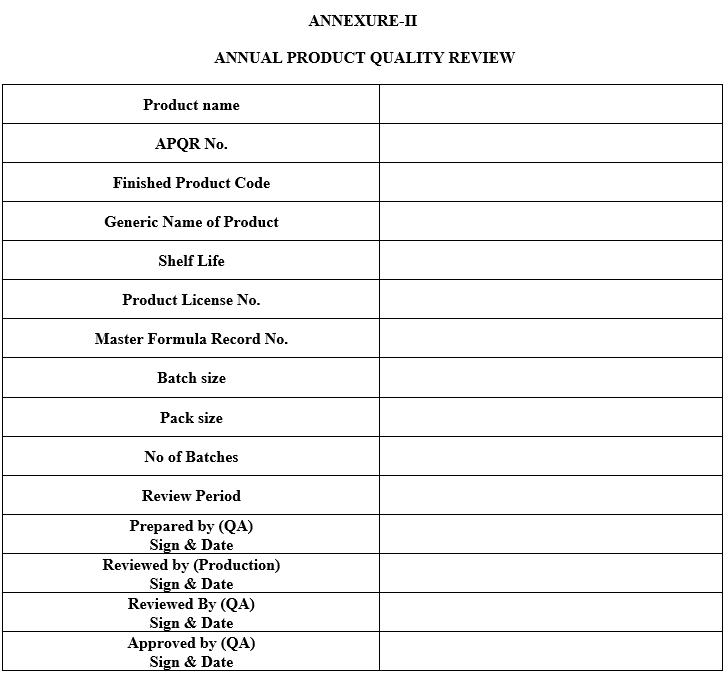
- Cover Page: Specify Product name (Different brand name with same Generic name), APQR No., Product Code, Generic Name of the product, Shelf Life, Product License No, Master Formula Record, Code, Batch Size, Pack Size, No of Batches, review period, prepared by, checked by and Approved by Sign date & Name.
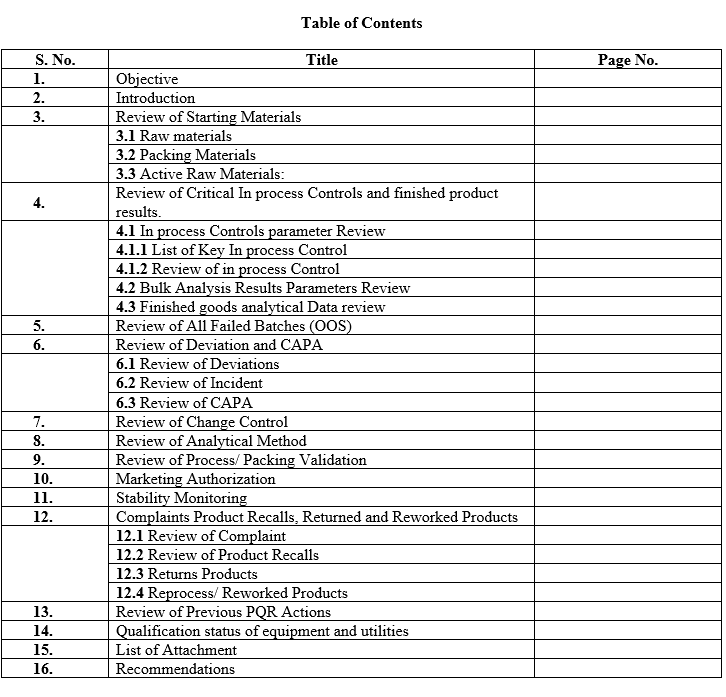
- Table of Contents: Record all content pages wise.
- Objective: Describe the objective.
- Introduction: Give in brief the history of the product like total no. of batches manufactured in the review period.
- A Review of starting materials and packaging materials used for the product, especially those from new sources, and particularly the review of supply chain traceability of active substances.
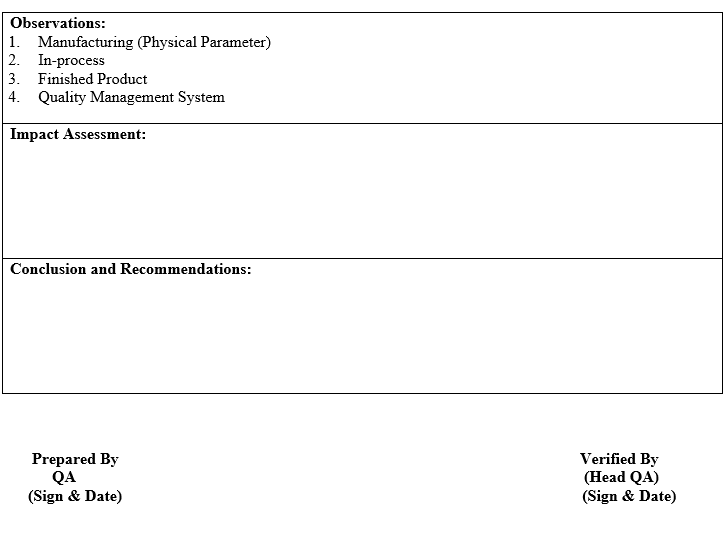
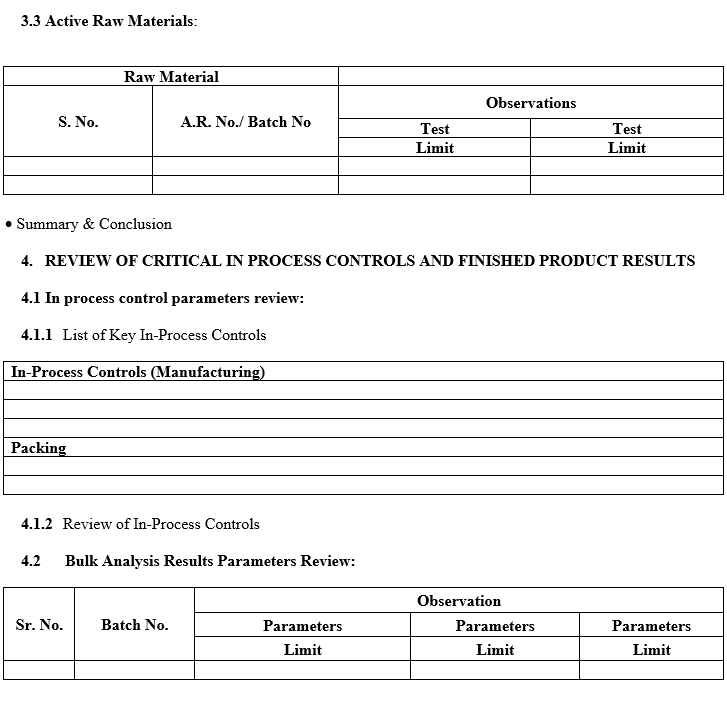
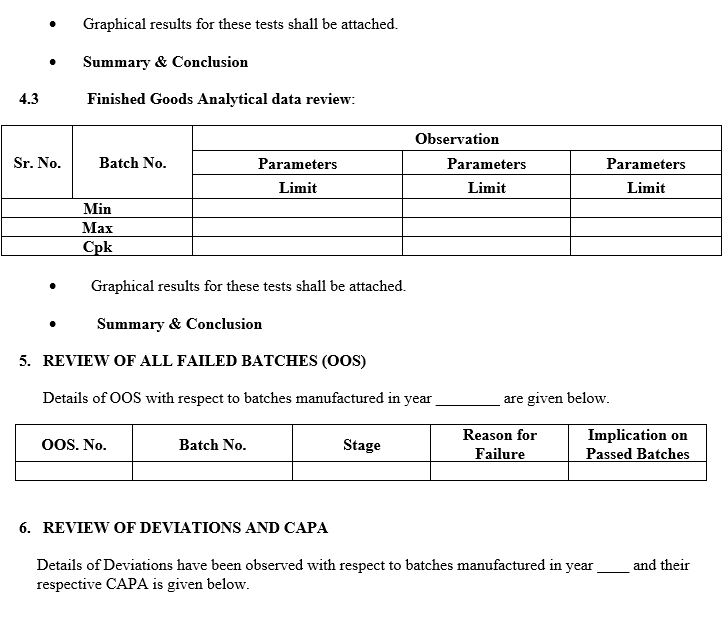
- A review of critical in-process controls and finished product results.
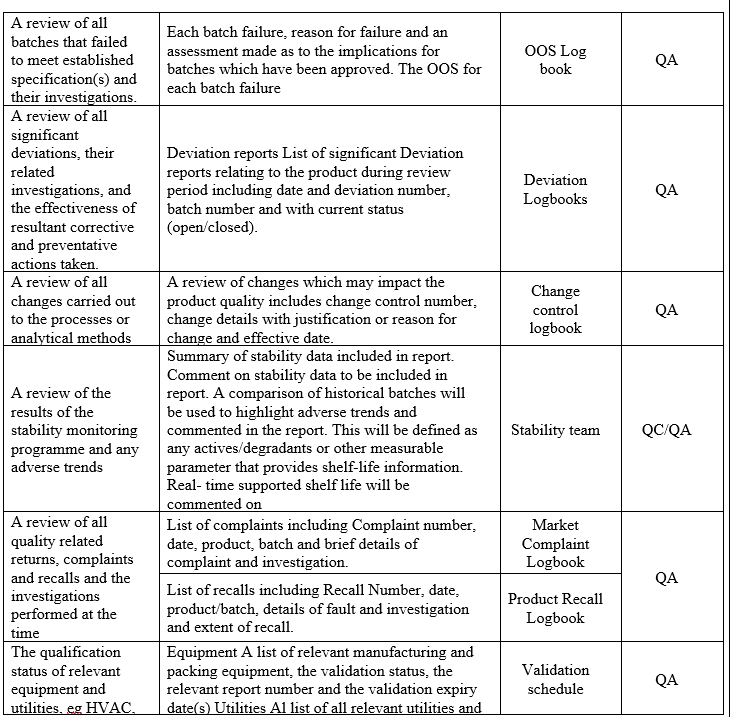
- A review of all batches that failed to meet established specification(s) and their investigation.
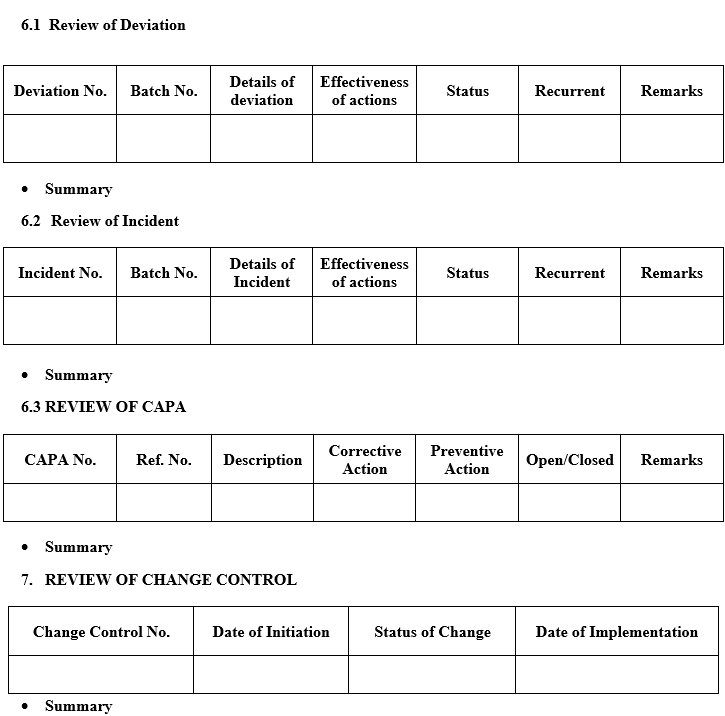
- A review of all Significant deviation and Incidents, related Investigations, and the effectiveness of resultant CAPAs taken.
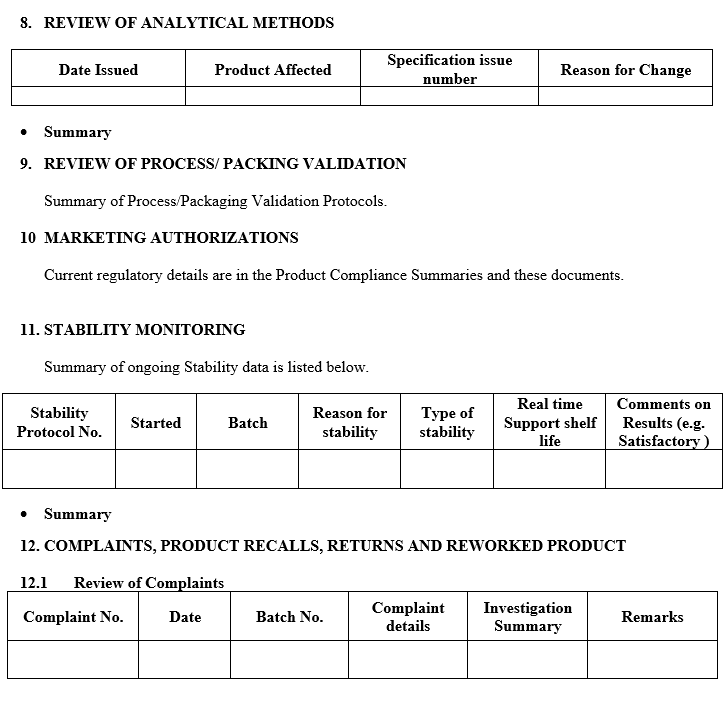
- A review of all changes made to the processes or analytical methods.
- A review of dossier Variations submitted, Granted or Refused.
- A review of the results of the stability monitoring programme and any adverse trends.
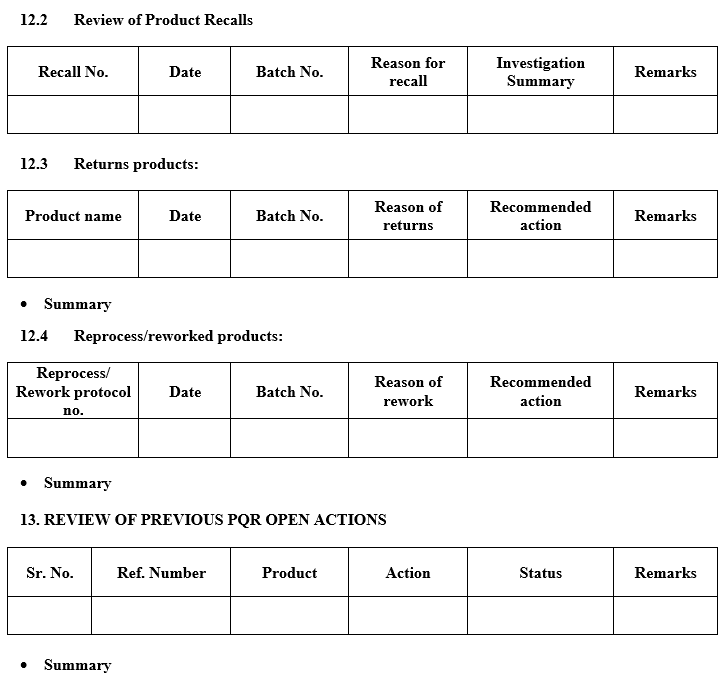
- A review of all quality-related returns, complaints and recalls and the investigations performed at the time.
- A review of adequacy of any other previous corrective actions on product processes or equipment.
- Post-marketing commitments for new dossiers and variations to the dossiers.
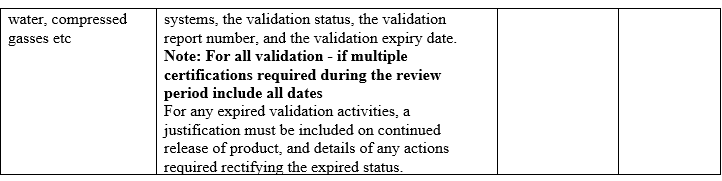
- The qualification status of relevant equipment and utilities, e.g. heating, ventilation, and air-conditioning (HVAC), water or compressed gases and a review of the results of monitoring the output of such equipment and utilities.
- A review of technical agreements to ensure that they are up to date.
- The review should cover the data and must note any actions required. These actions must be monitored via deviation. If any critical issues are noted, they will be immediately escalated to Plant Quality and QP who will act accordingly.
- Application of statistical tools for trending and reporting Quality parameters:
- Various statistical tools can be applied for reporting quality parameters during APQR. Some of the statistical tools which can be used for purpose are:
Cpk Value Control chart
Histogram
- Cpk value: Statistical tools of Process Capability Index (Cpk) shall be applied for evaluation of process parameters. Cpk Values below 1.0 shall be recommended for improvement. The Cpk Values between 1.0 & 1.33 shall be recommended for further trending during APQR of next year. Whereas Cpk values higher than 1.33 have been meant that the process parameter is rugged, and the manufacturing process is Capable for producing product with respect to said parameters.
- Definition: Process capability index (Cpk) is the measure of process capability. It shows how closely a process is able to produce the output to its overall specifications.
- Control Chart: This is used for graphical presentation of quality parameters of the product. It helps to reduce process variability. It helps to determine what kind of corrective action should be taken.
- Histogram: This bar chart is used for numerical categories. Its shape provides form of distribution of data. In this chart, Central tendency and variability can be easily estimated.
- Following data is required for the preparation of APQR describe the summary as per Annexure –II
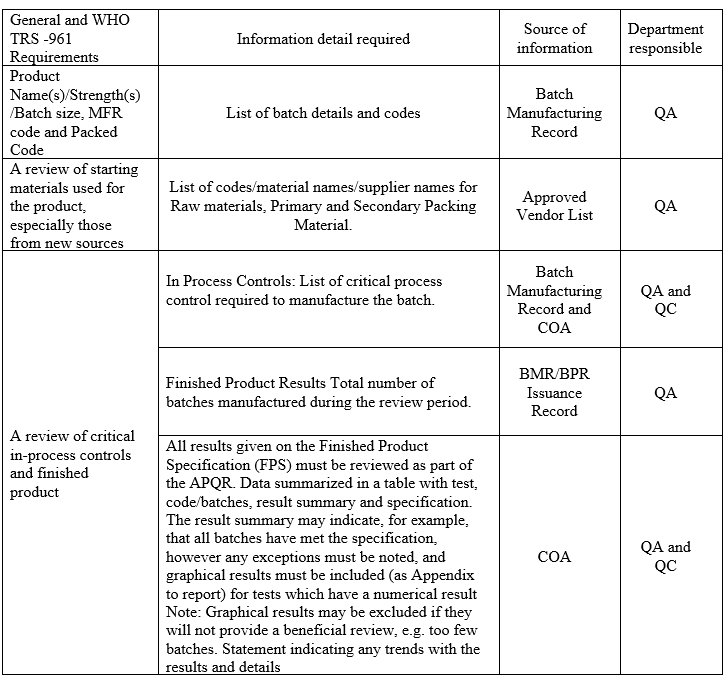
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/annual-product-quality-review/
- A review of critical in-process controls and finished product results shall be compiled product-wise or Batch No. wise. Any Out of trend (OOT) results obtained also shall be discussed in the report.
- In the case of Tablets, parameters such as Average weight, thickness, Hardness, Disintegration Test, Dissolution, LOD/Water content, Assay and Yield shall be reviewed as product specification.
- In the case of capsules, parameters such as Average fill weight, Disintegration Test, Dissolution, LOD/Water content, and Yield shall be reviewed as product specification.
- Data for trending and review shall be collected from relevant documents like Batch records, COA, Logbooks, Analytical test data sheets.
- If any abnormal trend of the data is observed during the compilation and review, it shall be commented on in the report.
- If any significant trend or abnormal observation is there in APQR, same shall be investigated as per relevant SOP.
- Continuous monitoring of Data: Designated QA person shall collect and compile data for product manufactured in routine for continuous monitoring.
- Designated QA person shall inform Head QA/ Designee if He/She find any discrepancies in results or trend.
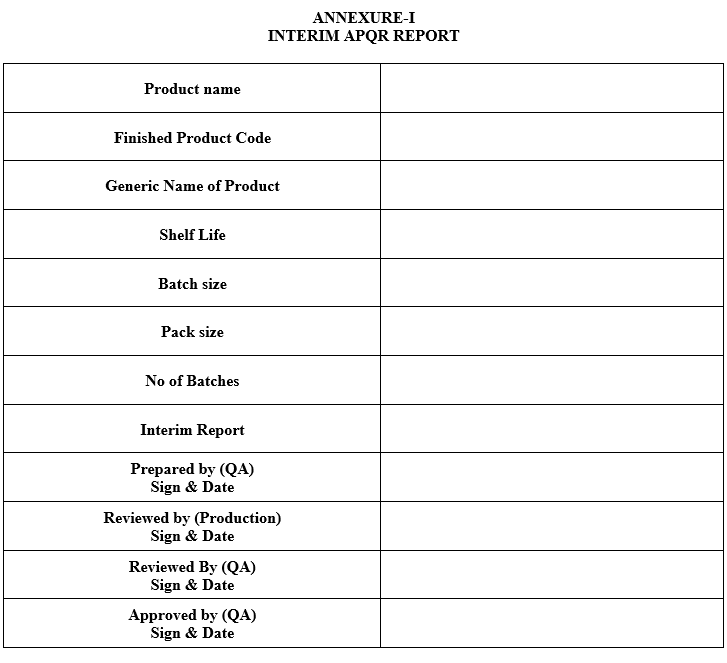
- An interim report shall cover in-process parameters, Finished Product COA, process validation & QMS observation. Data collected for “Interim APQR report” shall be done as per Annexure-I
- REFERENCES:
WHO TRS-961 Guideline on Product Quality Review
- ANNEXURES:
| ANNEXURE No. | TITLE OFANNEXURE |
| Annexure-I | Interim APQR Report |
| Annexure-II | Template for APQR |
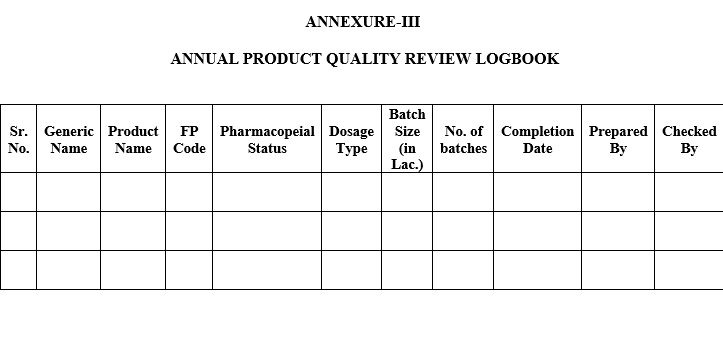
| Annexure-III | Logbook for APQR Report |
- DISTRIBUTION:

- ABBREVIATION:
| QA | : | Quality Assurance |
| SOP | : | Standard Operating Procedure |
| APQR | : | Annual Product Quality Review |
| OOS | : | Out of Specification |
| OOT | : | Out of Trend |
| No. | : | Number |
| LCL | : | Lower Control Limit |
| UCL | : | Upper Control Limit |
| CAPA | : | Corrective And Preventive Action |
| F&D | : | Formulations and Development |
| GMP | : | Good Manufacturing Practice |
| GLP | : | Good Laboratory Practice |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE-I
INTERIM APQR REPORT
ANNEXURE-II
ANNUAL PRODUCT QUALITY REVIEW

ANNEXURE-III
ANNUAL PRODUCT QUALITY REVIEW LOGBOOK
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/annual-product-quality-review/
Frequently Asked Questions?
Q: What is an Annual Product Quality Review (APQR) in the pharmaceutical industry?
A: An Annual Product Quality Review (APQR) is a comprehensive assessment conducted by pharmaceutical companies to evaluate the quality and compliance of their products over the course of a year. It is a regulatory requirement to ensure that pharmaceutical products consistently meet the required quality standards.
Q: Why is APQR important in the pharmaceutical industry?
A: APQR is crucial for ensuring the ongoing quality and safety of pharmaceutical products. It helps identify trends, deviations, and potential risks in the manufacturing process, enabling timely corrective and preventive actions. It also demonstrates a commitment to regulatory compliance and product quality improvement.
Q: What does the APQR process involve?
A: The APQR process involves a systematic review of various aspects, including production data, analytical results, complaints, recalls, deviations, changes in the manufacturing process, and stability data. It requires collaboration among different departments, such as quality control, production, regulatory affairs, and quality assurance.
Q: Who is responsible for conducting the APQR?
A: The responsibility for conducting the APQR lies with the pharmaceutical quality assurance department. This department ensures that the review is comprehensive, timely, and in compliance with regulatory requirements.
Q: What kind of data is typically included in an APQR?
A: An APQR includes data on production volumes, batch records, deviations, complaints, stability studies, changes in manufacturing processes, and any corrective and preventive actions taken during the reporting period. The data is analyzed to identify patterns or trends that may impact product quality.
Q: How often should APQRs be conducted?
A: APQRs are typically conducted annually, as the name suggests. However, companies may choose to conduct more frequent reviews based on the complexity of their manufacturing processes or regulatory requirements.
Q: What are the regulatory guidelines for APQR?
A: Regulatory guidelines for APQR vary by region, but they are commonly outlined by health authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other national regulatory agencies. Companies must follow these guidelines to ensure compliance.
Q: What happens if issues are identified during the APQR?
A: If issues are identified during the APQR, companies are required to implement corrective and preventive actions (CAPA) to address the root causes. These actions may include process improvements, changes in procedures, or other measures to ensure ongoing product quality.
Q: How does APQR contribute to continuous improvement in the pharmaceutical manufacturing process?
A: APQR contributes to continuous improvement by identifying areas for enhancement in the manufacturing process. The analysis of data allows companies to implement changes that can lead to increased efficiency, reduced deviations, and overall improvement in product quality.
Q: Can APQR findings impact product registration and market approval?
A: Yes, APQR findings can impact product registration and market approval. Health authorities may review APQR data during inspections, and deficiencies in the review process or product quality may affect regulatory approval or lead to regulatory actions.
Q: How should companies maintain documentation related to APQR?
A: Companies should maintain thorough documentation of the APQR process, including data sources, analysis methodologies, findings, and actions taken. This documentation is subject to regulatory inspection and should be kept in accordance with Good Documentation Practices (GDP).
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/annual-product-quality-review/