- OBJECTIVE:
To lay down a procedure for the identification and classification of GMP Deficiencies in Pharmaceutical Industry.
- SCOPE:
This SOP is applicable for the identification and classification of GMP Deficiencies in Pharmaceutical Industry at {Company Name} {Location}.
- RESPONSIBILITY:
- Production: To identify the deficiency and non-compliance during production and packing process and provide a CAPA to prevent occurrence.
- Quality Assurance: To review the identified deficiency and provide and approve a CAPA to prevent occurrence.
- Quality Control: To identify the deficiency and non-compliance during quality check and provide a CAPA to prevent occurrence.
- Engineering: To identify the deficiency and non-compliance during engineering work and provide a CAPA to prevent occurrence.
- Warehouse: To identify the deficiency and non-compliance during the receiving, issuing and storage of material and provide a CAPA to prevent occurrence.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- The GMP Deficiencies findings of a Good Manufacturing Practice (GMP) inspection can have a substantial impact on both your organization and, subsequently, public health.
- If the GMP Deficiencies found are classified as significant, they may necessitate cessation of manufacturing activities and/or product withdrawal from the market.
- In the advent of globalization, harmonized procedures, including the classification of GMP Deficiencies, will improve inter-agency consistency and provide transparency throughout the industry.
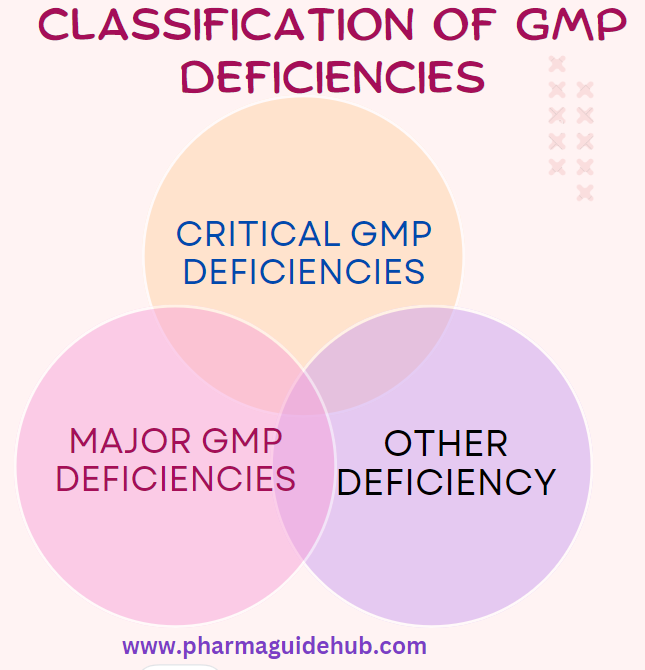
- CRITICAL GMP DEFICIENCIES:
- A GMP Deficiencies that has produced, or leads to a significant risk of producing either a product that is harmful to the patient.
- A “Critical” GMP Deficiencies also occurs when it is observed that the manufacturer has engaged in fraud, misrepresentation, or falsification of products or data.
- A “Critical” GMP Deficiencies may consist of several related deficiencies, none of which on its own may be “Critical”, but which may together represent a” Critical” deficiency or systems’ failure where a risk of harm was identified and should be explained and reported as such.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/classification-of-gmp-deficiencies-in-pharmaceutical-industry/
Critical Deficiency Examples A “Critical” GMP Deficiencies is a serious situation that could result in regulatory action being considered.
- Lack of sterilization validation (relevant to all sterile products).
- Lack of adequate control measures resulting in an actual, or significant risk of, cross-contamination above the level of the health-based exposure limit in subsequent products.
- Evidence of gross pest infestation (relevant to all manufacturers).
- Falsification or misrepresentation of analytical results or records (relevant to all manufacturers).
- Failure to ensure the quality and/or identity of starting materials (relevant to all manufacturers).
- No master batch documents (relevant to all manufacturers).
- Absence, falsification, or misrepresentation of manufacturing and packaging records (relevant to all manufacturers).
- The water system for sterile products is not validated (for manufacturers of sterile products).
- HVAC system for sterile products not validated (for manufacturers of sterile products).
- Grossly unsuitable premises so that there is a high or likely risk of contamination (relevant to all manufacturers).
- No evidence that mandated recall processes has been complied with (relevant to all manufacturers).
- MAJOR GMP DEFICIENCIES: A GMP Deficiencies that is not a “Critical” deficiency, but which:
- Has produced or may produce a product that does not comply with its Marketing Authorisation, Clinical Trial Authorisation, product specification; pharmacopeia requirements, or dossier.
- Does not ensure effective implementation of the required GMP control measures.
- Indicates a major deviation from the terms of the manufacturing authorization.
- Indicates a failure to carry out satisfactory procedures for the release of batches or failure of the authorized person to fulfil his/her duties.
- Consists of several “Other” related GMP Deficiencies, none of which on its own may be “Major”, but which may together represent a “Major” GMP Deficiencies or systems failure and should be explained and reported as such.
Some Examples of GMP Deficiencies rated as “Major” (in the absence of risk-reducing factors) include the following:
- Lack of validation of critical processes (applicable to all medicines, but could be “Critical” for low-dose/high-potency products; particularly sterilization processes for sterile products)
- No or grossly inadequate air filtration to minimize airborne contaminants (applicable to all medicines manufacturers – could be “Critical” if possible, contaminants are a safety concern and “Critical” for sterile medicines)
- Missing or ineffective control measures to provide adequate confidence that cross-contamination will be controlled within the health-based exposure limit in subsequent products. (would be “Critical” if resulting cross-contamination has or is likely to exceed the health-based exposure limit).
- Damage (holes, cracks, peeling paint) to walls/ceilings in manufacturing areas where the product is exposed in non-sterile areas.
- Design of manufacturing area that does not permit effective cleaning.
- Insufficient manufacturing space could lead to mix-ups.
- No raw material sampling area for medicine manufacturers (could be classed as “Other” if adequate precautions are taken).
- Sanitary fittings are not used on liquid/cream manufacturing equipment.
- Stored equipment is not protected from contamination.
- Individuals in charge of QC/production are not qualified by education, competency training, and experience.
- Inadequate initial and ongoing training and/or no training records.
- Cleaning procedures not documented and/or no cleaning records.
- Production equipment cleaning procedures not validated.
- Reduced QC testing of raw materials without data to certify suppliers.
- Incomplete testing of raw materials.
- Test methods not validated.
- Complex production processes for non-critical products not validated.
- Unapproved/undocumented changes to master batch or equivalent documents.
- Deviations from instructions not approved.
- No or inadequate internal inspection program.
- No proper release for the supply procedure.
- The product was reworked without proper approval.
- No system/procedures for handling complaints or returned goods.
- Inadequate testing of packaging materials.
- No ongoing stability program and/or stability data for all products not available.
- Insufficient lighting in production or inspection areas.
- The containers from which samples have been taken were not identified.
- The temperature of critical temperature-controlled storage areas is not monitored or alarmed.
- Inadequate change control system.
- Inadequate deviation system.
- No investigation into alarms and temperature excursions for deviations from storage or transport requirements.
- OTHER DEFICIENCY: A deficiency that is not classified as either “Critical” or “Major”, but indicates a departure from Good Manufacturing Practice (GMP).
- A deficiency may be judged as “Other” because there is insufficient information to classify it as “Critical” or “Major”.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/classification-of-gmp-deficiencies-in-pharmaceutical-industry/
- REFERENCES:
PIC/s guidance on classification of GMP deficiencies (PI 040-1)
- ANNEXURES:
Not Applicable
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Production
- Controlled Copy No. 03 : Head Quality Control
- Controlled Copy No. 04 : Head warehouse
- Controlled Copy No. 05 : Head Engineering
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| GMP | : | Good Manufacturing Practices |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| CAPA | : | Corrective And Preventive Actions |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Frequently Asked Questions?
Q: What potential impact do GMP Deficiencies in a Good Manufacturing Practice (GMP) inspection have?
A: GMP Deficiencies found in a GMP inspection can have a substantial impact on both the organization and public health.
Q: What actions might be necessitated if GMP Deficiencies are classified as significant?
A: If GMP Deficiencies are classified as significant, they may require the cessation of manufacturing activities and/or the withdrawal of products from the market.
Q: How can harmonized procedures, including the classification of GMP Deficiencies, benefit the pharmaceutical industry?
A: Harmonized procedures, including the classification of GMP Deficiencies, in the era of globalization, enhance inter-agency consistency and provide transparency throughout the pharmaceutical industry.
Q: How are Critical GMP Deficiencies defined?
A: Critical GMP Deficiencies are those that either produce or pose a significant risk of producing a product harmful to the patient. They also occur when fraud, misrepresentation, or falsification of products or data is observed. Multiple related deficiencies, individually not critical, may collectively represent a Critical deficiency or systems failure.
Q: Provide examples of Critical GMP Deficiencies.
A: Examples of Critical GMP Deficiencies include lack of sterilization validation, inadequate control measures leading to a risk of cross-contamination, evidence of gross pest infestation, falsification or misrepresentation of analytical results, failure to ensure quality and identity of starting materials, absence of master batch documents, and more.
Q: What are the characteristics of Major GMP Deficiencies?
A: Major GMP Deficiencies, while not critical, may produce a product not in compliance with its authorization, specification, or pharmacopeia requirements. They indicate deviations from the manufacturing authorization terms, failure in implementing required GMP control measures, and may consist of several related deficiencies representing a Major GMP Deficiency or systems failure.
Q: Provide examples of Major GMP Deficiencies.
A: Examples of Major GMP Deficiencies include lack of validation of critical processes, inadequate air filtration, missing or ineffective control measures for controlling cross-contamination, damage to walls/ceilings in manufacturing areas, insufficient manufacturing space leading to mix-ups, inadequate training, incomplete testing of raw materials, and more.
Q: What is an “Other Deficiency” in the context of GMP?
A: An “Other Deficiency” is a deficiency that is not classified as either “Critical” or “Major” but indicates a departure from Good Manufacturing Practice (GMP).
Q: Why might a deficiency be classified as “Other”?
A: A deficiency may be classified as “Other” when there is insufficient information to categorize it as “Critical” or “Major.”
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/classification-of-gmp-deficiencies-in-pharmaceutical-industry/