- OBJECTIVE:
To lay down a procedure for additional page issuance of controlled documents.
- SCOPE:
This SOP is applicable for the additional page issuance of controlled documents like BMR, BPR & formats at {Company Name} {Company Location}.
- RESPONSIBILITY:
- QC, Micro, Production, Engineering, Warehouse, and HR Officer/Executive: Follow the instructions as per procedure.
- The QA Executive/Designee is responsible for the preparation of this SOP.
- QA Sr. Executive/Designee responsible for Review and technical correction of SOP.
- ACCOUNTABILITY:
The QA Head shall be accountable for the approval of the SOP.
- PROCEDURE:
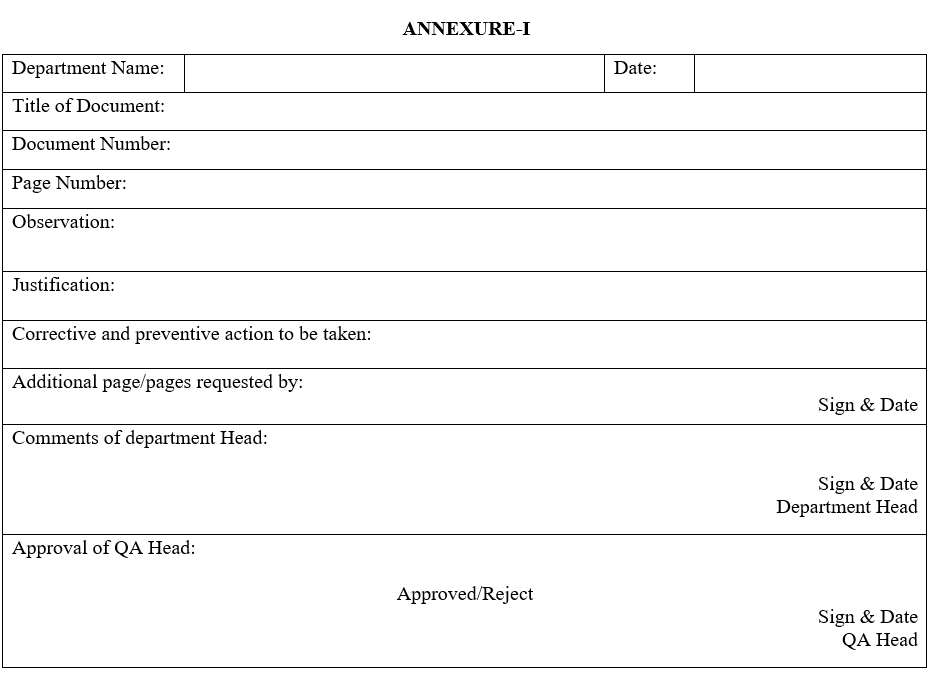
- As per the requirement of additional page/pages in the controlled document, the respective department officer/executive shall request to QA for issue the Request Form for Additional Pages (As per Annexure-I).
- The respective department officer/executive shall take the comment and signature of the respective department head.
- After taking the signature and comment of department head/Designee, request a form for additional pages (as per Annexure-I) and send it to the QA head for approval.
- After the approval of the QA head/Designee, QA officer/executive shall take the required number of photocopies from the master document and issue them to the respective department and fill the required detail in the document/Format issuance, retrieval and destruction log of relevant SOP.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/additional-page-issuance-of-controlled-documents/
- REFERENCES:
Not Applicable.
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Request Form for Additional Pages |
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Controlled Copy No. 03 : Head Production
- Controlled Copy No. 04 : Head Engineering
- Controlled Copy No. 05 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| QA | : | Quality assurance |
| SOP | : | Standard operating procedure |
| No. | : | Number |
| HR | : | Human Resources |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE-I
Request Form for Additional Pages
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/additional-page-issuance-of-controlled-documents/
Frequently Asked Questions?
1. Question: Why is there a need for additional page issuance of controlled documents in the pharmaceutical industry?
Answer: The additional page issuance of controlled documents in the pharmaceutical industry is necessary to accommodate revisions, updates, or new information that arises after the initial issuance. This ensures that the documents remain accurate and compliant with regulatory requirements.
2. Question: What types of controlled documents typically require additional pages in the pharmaceutical setting?
Answer: Controlled documents, such as batch records and validation protocols often require additional pages. Any document critical to compliance, quality, and safety may undergo updates or changes, warranting the issuance of additional pages.
3. Question: How does the pharmaceutical industry ensure the controlled nature of additional pages issued for documents?
Answer: To maintain control, the pharmaceutical industry follows established document control procedures. Each additional page is assigned a unique identifier, and a comprehensive version control system ensures proper tracking, review, approval, and distribution.
4. Question: What is the significance of version control in the context of additional page issuance for controlled documents?
Answer: Version control is crucial to tracing the evolution of a document. It ensures that stakeholders are working with the latest and approved information, minimizing the risk of errors and non-compliance.
5. Question: How often should pharmaceutical companies review and update controlled documents, prompting the issuance of additional pages?
Answer: The frequency of document reviews varies based on regulatory requirements and internal quality management systems. Typically, updates are triggered by changes in processes, equipment, regulations, or lessons learned from deviations.
6. Question: How are additional pages for controlled documents approved in the pharmaceutical industry?
Answer: The approval process involves a thorough review by subject matter experts, quality assurance personnel, and relevant stakeholders. Once approved, the new pages are integrated into the existing document, and the updated version is reissued.
7. Question: What measures are in place to prevent unauthorized issuance or alterations of additional pages for controlled documents?
Answer: Access controls, electronic signatures, and audit trails are implemented to prevent unauthorized changes. Only authorized personnel with the appropriate training and permissions can initiate, review, approve, and distribute additional pages.
8. Question: How does the issuance of additional pages impact training and awareness within pharmaceutical companies?
Answer: Training programs are essential to educate employees on the changes introduced through additional pages. Awareness campaigns ensure that staff members are informed about the revisions and understand their implications on daily operations.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/additional-page-issuance-of-controlled-documents/





