- PROCEDURE:
- ARD will send prerequisite for method transfer. The QC Manager will send it back after approval.
- Specification and STP for the analytical method will be issued by ARD to QC Laboratory.
- Time line will be decided by mutual discussion between ARD and QC.
- The QC laboratory will review the list required for method transfer and procure all necessary items.
- The Head, ARD or a person designated by him will decide on the option of the collaborative Transfer or direct transfer in discussion with the in charge QC.
- As per the ARD protocol and STP, QC shall prepare work sheets for method transfer. Work sheets shall be prepared as per SOP.
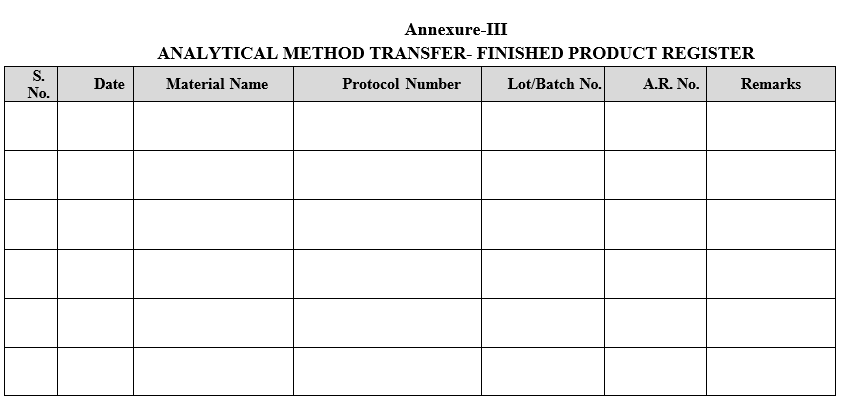
NOTE: All Analytical techniques are not covered under guideline/acceptance criteria. The plan and acceptance criteria for these will be decided by ARD and a proper justification will be give in the protocol.
- In case of Collaborative Transfer, ARD analyst and QC analyst will perform analysis on the same batch as per protocol in ARD and QC laboratory respectively.
- In case of Direct Transfer, person designated by Head, ARD will visit QC Laboratory and demonstrate the experimental tips and precautions, to the person designated by the QC – Head.
- Both ARD analyst and QC analyst will perform analysis as per protocol in the QC Laboratory.
- Samples for method transfer shall be register as per Format-II and Format-III.
- Analytical Report Number shall be allot as per SOP.After completion of testing, analyst shall enter the results and sign with date in all Work sheets and completed worksheet and chromatograms shall be handed over to Section-in-charge.
- The work sheets, calculations, chromatograms, spectra’s, and the analytical report shall be thoroughly verified by Senior Executive- QC with sign and date as per SOP.
- Analytical data shall be compare in Method Transfer report.
- Method Transfer Report Numbering System
- Method Transfer Report Number consists AMTR/P/DD/XX/YY-NNN
| AMTR | : | Analytical Method Transfer Report |
| / | : | slash |
| P | : | Pharmaguidehub |
| / | : | slash |
| DD | : | Department name Quality Control |
| / | : | slash |
| XX | : | Raw Material/Finished Product. (i.e.: RM/FP) |
| / | : | slash |
| YY | : | Year |
| – | : | Dash |
| NNN | : | three digits serial number starting from 001,002 and so on. |
- For e.g.:
- First Analytical Method Transfer Report for Raw material of 2025 shall be given as AMTR/P/QC/RM/25-001.
- First Analytical Method Transfer Report for Finished Product of 2025 shall be given as AMTR/P/QC/FP/25-001.
- The Method transfer report along with supporting data shall be submitted to QA department.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Guidelines and Acceptance Criteria for Method transfer Protocol |
| Annexure-II | Analytical Method transfer- Raw material Register |
| Annexure-III | Analytical Method transfer- Finished product Register |
Annexure-I
GUIDELINES AND ACCEPTANCE CRITERIA FOR METHOD TRANSFER PROTOCOL
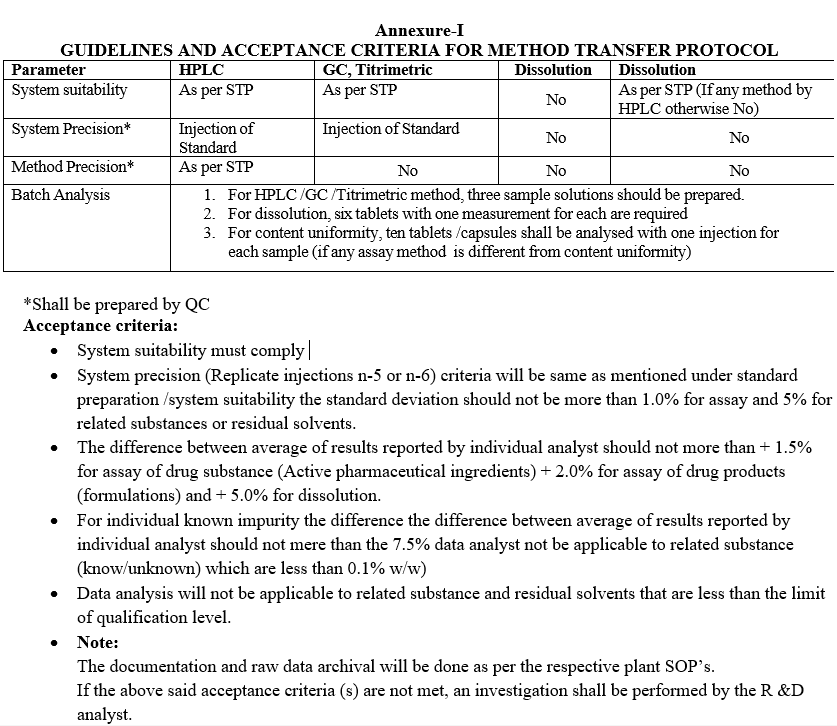
Annexure-II
ANALYTICAL METHOD TRANSFER- RAW MATERIAL REGISTER
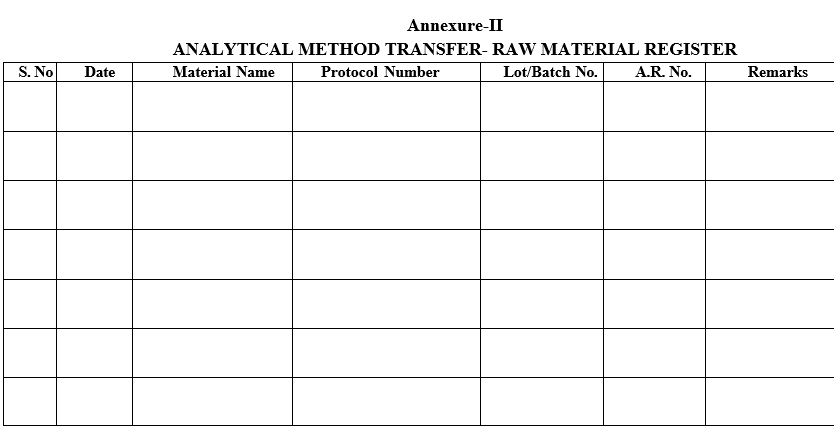
Annexure-III
ANALYTICAL METHOD TRANSFER- FINISHED PRODUCT REGISTER