- OBJECTIVE:
- To lay down a procedure for analytical work sheet and COA preparation.
- SCOPE:
This SOP is applicable to the procedure for analytical work sheet and COA preparation at {Company Name} {Location}.
- RESPONSIBILITY:
- Officer/Executive/Designee Quality Control – Shall be responsible for operation as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- QA Executive/Designee – Issuance and retrieval of work sheets.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- The following procedure shall be considered for preparation & issuance of work sheet and COA:
- All worksheets shall be prepared as per STP’s with accuracy and correctness of data for the tests and the approved worksheets shall be issued to QC.
- All the work sheets shall be issued by QA personnel.
- All the worksheets shall be issued to QC by QA stamp and sign with date.
- All COA’s shall be prepared as per specification with accuracy and correctness of data for the tests, respectively for Finished product and Raw material.
- QC shall fill requisition of analytical work sheet issuance.
Note: Only Approved Worksheets shall be issued.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/analytical-work-sheet-and-coa-preparation/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
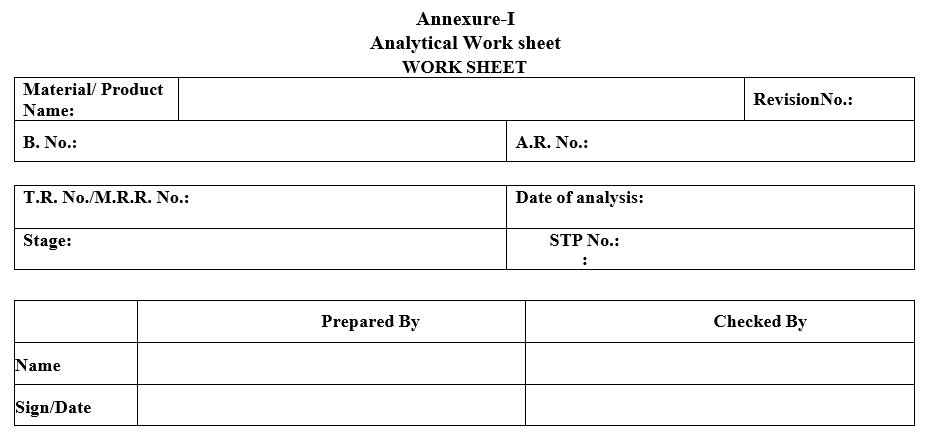
| Annexure-I | Analytical Work Sheet |
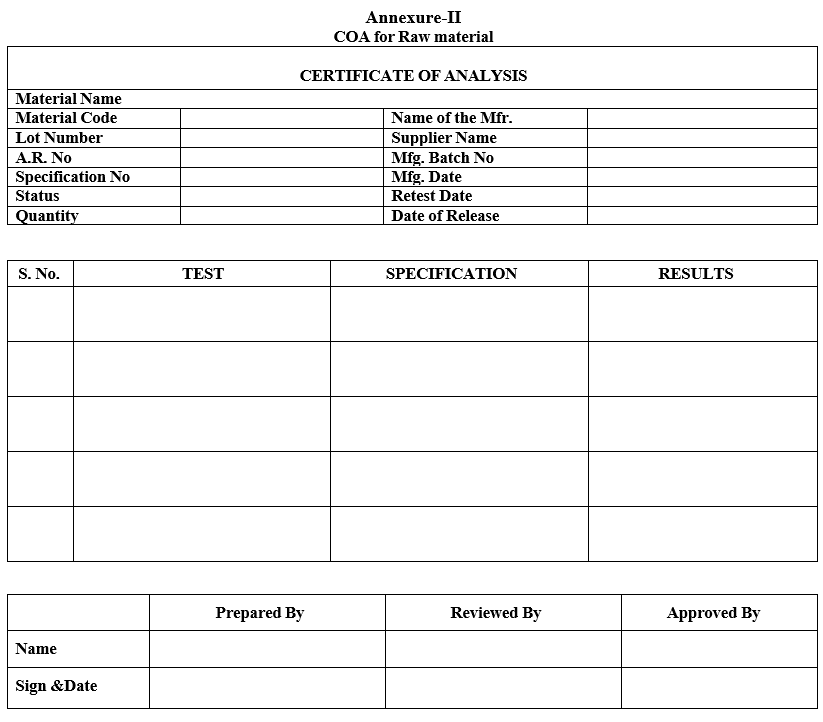
| Annexure-II | COA of Raw material |
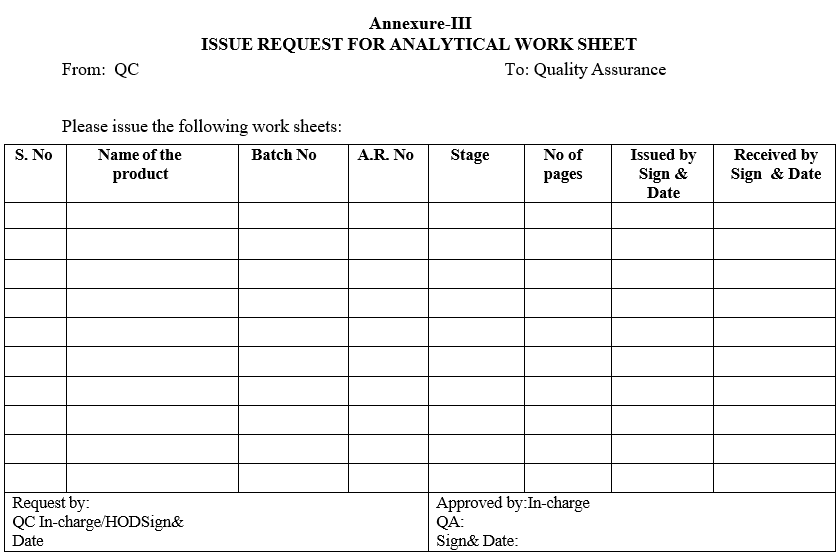
| Annexure-III | Issue request for analytical work sheet |
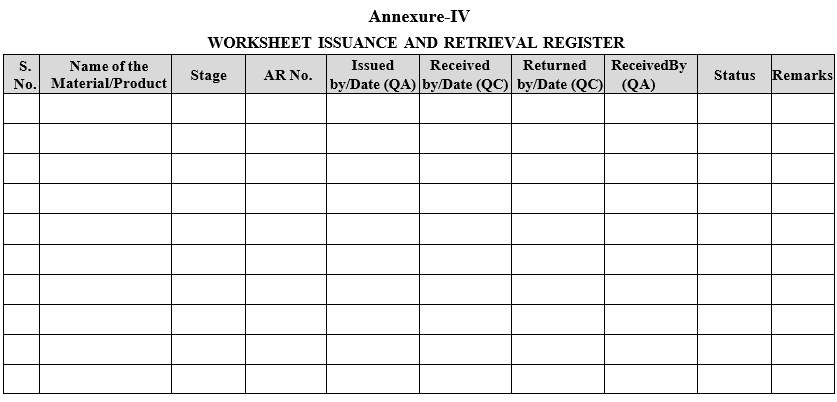
| Annexure-IV | Worksheet issuance and retrieval register |
| Annexure-V | COA of finished product |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| COA | : | Certificate of analysis |
| STP | : | Standard test procedures |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Analytical Work sheet
WORK SHEET
Annexure-II
COA for Raw material
Annexure-III
ISSUE REQUEST FOR ANALYTICAL WORK SHEET
Annexure-IV
WORKSHEET ISSUANCE AND RETRIEVAL REGISTER
Annexure-V
CERTIFICATE OF ANALYSIS OF FINISHED PRODUCT
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/analytical-work-sheet-and-coa-preparation/
