- OBJECTIVE:
To lay down a Procedure for Batch Conversion.
- SCOPE:
This SOP is applicable for Batch Conversion at {Company Name} {Company Location}.
- RESPONSIBILITY:
- User Department: Initiator & Requisition for batch conversion note.
- QA Officer / Executive/ User Department shall be responsible for Follow the instruction as per Procedure.
- QA Head – Training, Approval, and implementation of SOP.
- ACCOUNTABILITY:
QA Head shall be accountable for Approval, Training, Implementation and Execution of this SOP
- PROCEDURE:
- Definition:
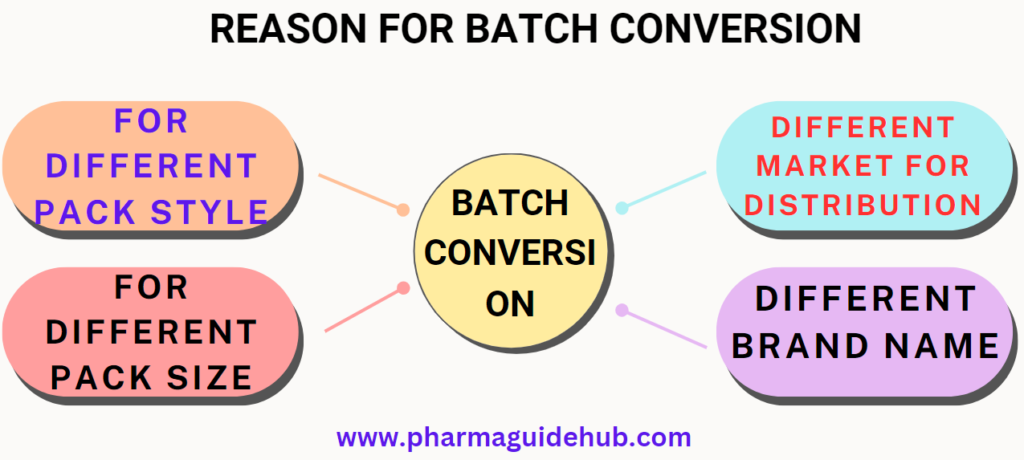
- Batch conversion: Mother Single batch convert into different batches, for example different pack style as physician sample and sale / Different Pack Size/ Different market for distribution/ Different brand Name.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/batch-conversion/
- Batch conversion shall be done when single batch is to be packed in:
- Different pack style as physician sample and sale.
- Different Pack Size
- Different market for distribution
- Different brand Name.
- The batch Conversion record shall be raised by PPIC /Production Department in following Conditions:
- Batch Conversion of sale to Export /PS & Domestic supply.
- Batch Conversion Shall also be done for Difference Packing Style (Bottle, Corton etc.)
- Batch Conversion can be carried out at semi-finished stage.
- Batch Conversion Shall be performed As Per Annexure I.
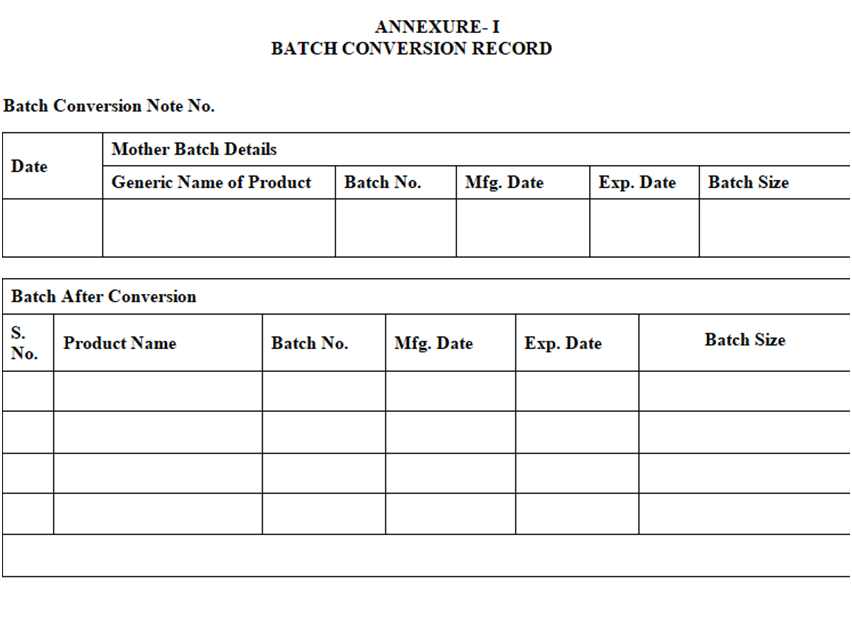
- The Annexure-I shall contain the following contents:
- Batch conversion Details in Batch conversion Record. Batch Conversion note No. Generic Name, Batch No. Mfg Date, Exp. Date and Batch Size.
- Batch Details After Conversion Product Name, Batch No. Mfg. Date, Exp. Date, Batch Size.
- A reference copy of the approved Batch Conversion Record shall be attached in all batch packing records including mother batches.
- In case of a market complaint due to quality in any of the converted batches or mother batches, all the batches shall be recalled.
Steps of Batch Conversion:
A request for issuance of Batch conversion note shall be initiated.
The record of issuance shall be Batch conversion note shall be recorded in format.
Batch conversion note numbering shall be assigned as:
BCN/YY/ZZZ
Where,
BCN: Indicates to Batch conversion note.
YY : Last two digit of the current year
ZZZ: Indicates to serial no start from 001, 002, 003……….
The Batch conversion note numbering for the first batch conversion in year 2024 is BCN/24/001
- A request shall be initiated for the issuance of Batch manufacturing Record & Batch Packing Record for recording the detail of operation of converted batches.
- The Batch Details of the batch conversion shall be checked by Production Head.
- After verification of batch conversion details the production Head shall forward the Batch Conversion Record to Head QA for approval.
- REFERANCE: Not Applicable
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/batch-conversion/
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Batch Conversion Record |
- DISTRIBUTION :
- Controlled Copy No.01 : Head Quality Assurance
- Controlled Copy No.02 : Head Production
- Controlled Copy No.03 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
QA : Quality Assurance
Mfg. : Manufacturing
PPIC : Production Planning & Inventory Control
- REVISION HISTORY :
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Changes | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE- I
BATCH CONVERSION RECORD
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/batch-conversion/
Frequently Asked Question ?
Q: What is batch conversion?
- Answer: Batch conversion is the process of taking a single batch of a product and dividing it into multiple batches with different characteristics. For example, a single batch of medication might be converted into different pack sizes for sale and physician samples.
Q: Why is batch conversion used?
- Answer: Batch conversion is used for various reasons, such as:
- Meeting different market demands: Different markets may have different packaging requirements or regulations.
- Optimizing inventory management: Different pack sizes can be used to meet varying demand levels or avoid having large quantities of product in one format.
- Introducing new brands: A single batch can be used to create multiple brands with different marketing strategies.
Q: When is batch conversion required?
- Answer: Batch conversion is required when a single batch needs to be packaged in different ways, such as:
- Different pack styles (bottles, cartons, etc.)
- Different pack sizes
- Different markets for distribution
- Different brand names
Q: Who initiates the batch conversion process?
- Answer: The PPIC (Production Planning and Inventory Control) or Production department raises the batch conversion record if the conditions mentioned above apply.
Q: Can batch conversion be done at different stages?
- Answer: Yes, batch conversion can be carried out at the semi-finished stage, before the final product is completed.
Q: How is the batch conversion process conducted?
- Answer: The process involves several steps, including:
- Initiating a request for a batch conversion note.
- Recording the request in a specific format.
- Assigning a unique numbering system to the batch conversion note.
- Submitting the note to the Quality Assurance department for review.
- Creating and issuing batch manufacturing and packing records for the converted batches.
- Verifying the details of the conversion by the Production Head.
- Forwarding the approved batch conversion record to the Head of Quality Assurance for final approval.
Q: What is included in the Batch Conversion Request document?
- Answer: Batch conversion request documents contains details about both the original (mother) batch and the converted batches, including:
- Batch conversion details
- Batch conversion note number
- Generic name of the product
- Batch number
- Manufacturing date
- Expiry date
- Batch size
- Product name after conversion
- Batch number after conversion
- Manufacturing date after conversion
- Expiry date after conversion
- Batch size after conversion
Q: What happens if there is a quality issue with a converted batch?
- Answer: In case of a market complaint due to quality issues in any of the converted batches or the mother batch, all affected batches are subject to recall.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/batch-conversion/




