
OBJECTIVE:
To lay down the procedure for Calibration of Media Dispenser, Make: Biotool.
SCOPE:
This SOP is applicable for the procedure for Calibration of Media Dispenser, Make: Biotool at {Company Name} {Location}.
RESPONSIBILITY:
Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
Microbiologist: is responsible to perform the activity as per SOP.
In charge- Microbiology- is responsible to ensure compliance as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
About Biotool Media Dispenser
Biotool’s media dispensers are efficient laboratory tools that automate the process of dispensing liquid media into Petri dishes or other containers. These dispensers offer precise control over the volume of media dispensed, ensuring consistent and reproducible results. They are designed to improve efficiency and reduce the risk of contamination in microbiological laboratories.
PROCEDURE:
Calibration of Media Pump:
Before you calibrated the pump, the tube must be filled with the medium. This can be made with the Manual mode. Effectively pumped quantity of culture medium can be calibrated in the Pump Calibration menu. Using the C-TEST key. Check after calibration is complete whether the volume is being dispensed precisely.
C-TEST can also be use to induct the medium. abort the process with STOP.
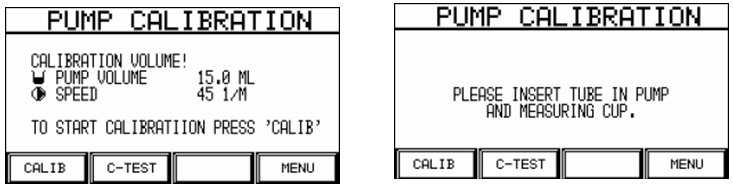
Procedure for calibrating the pump volume:
After starting calibration, the user is prompted to insert the tube into the pump and measuring beaker.
The calibration is dispensed into the measuring beaker after restart.
After completion of pumping, the pumped volume must be measured (e.g. with measuring beaker or scales…) and the result must be entered on the numeric keypad.
Pump calibration can be completed with the Enter Key, thus saving the results.
The calibrated pump volume is taken as the basis for all volume and speed changes after a calibration.
Note: Frequency of calibration every 6 months/whenever required
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/calibartion-of-media-dispenser-make-biotool/
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration Report of Media Dispenser Pump |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control (Micro.)
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
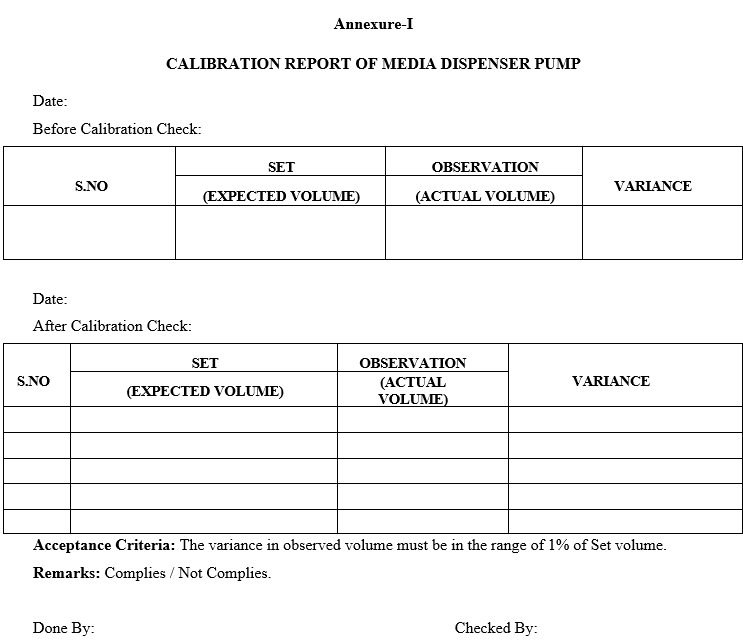
Annexure-I
CALIBRATION REPORT OF MEDIA DISPENSER PUMP
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/calibartion-of-media-dispenser-make-biotool/
