- OBJECTIVE:
To lay down the procedure for Calibration of auto titrator and electrodes.
- SCOPE:
- This SOP is applicable to the procedure for Calibration of auto titrator and electrodes at {Company Name} {Location}.
- RESPONSIBILITY:
- Officer/Executive/Designee Quality Control – Shall be responsible for calibration of instrument as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
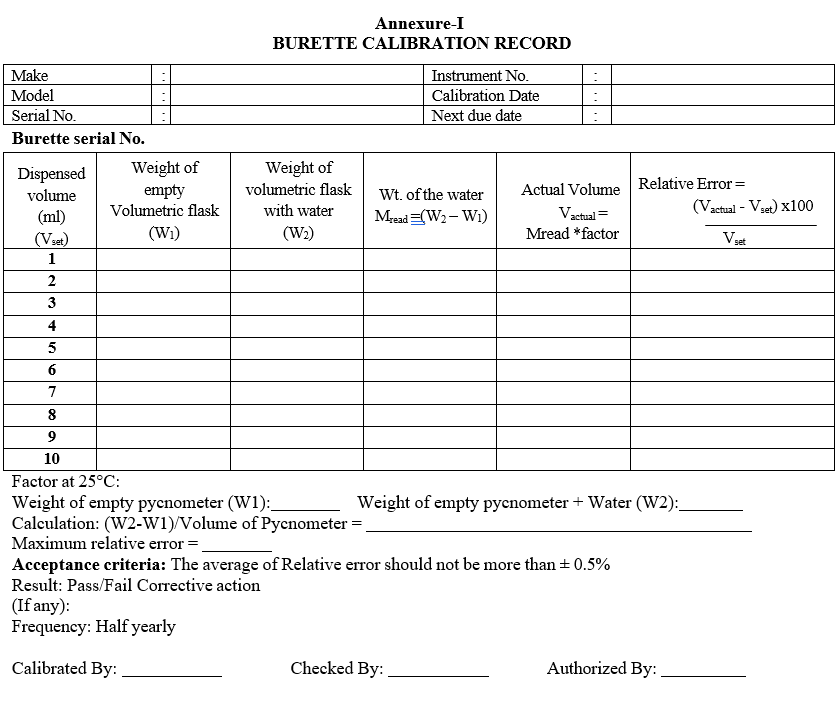
- Calibration of burette:
- Operate the instrument as per Sop, Rinse well the burette and fill the Burette with purified water.Take a clean, dry 10ml volumetric flask.
- Weigh the volumetric flask and note down the weight as W1.
- Set the dispense volume as 1 ml and dose the water into the Volumetric flask.
- Weigh the volumetric flask with water and note weight as W2.
- Repeat the above 3 steps for volumes 2,3,4,5,6,7,8,9,10 respectively.Mread = W2 – W1
- Vactual = Mread x Density
- Calculate the relative error using the formula
- Relative error = (Vactual –Vset) x 100/Vset
- Density calculation:
- Take a pycnometer and note down the empty weight (W1).
- Fill the pycnometer with water, close the stopper provided and wipe off the excess water.
- Now note down the weight of pycnometer with water at 25°C (W2).
- Weight per ml of water = (W2-W1)/Volume of Pycnometer.
- Enter the details of calibration in calibration record as per format-I.
- Acceptance criteria: the average of Relative error should not be more than ± 0.5%
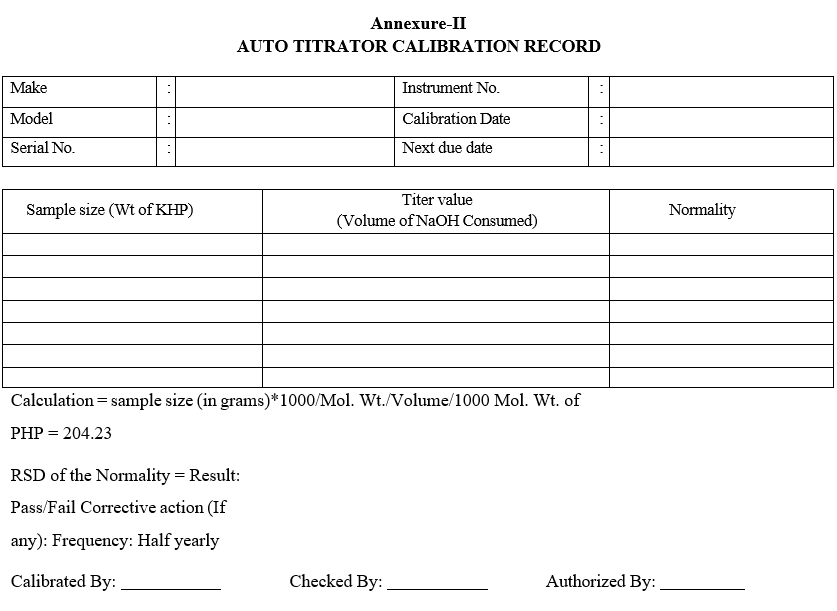
- Auto titrator calibration:
- Select a standard titration (KHP versus NaOH) suggested by Metrohm.Prepare 0.1M NaOH solution and perform the standard titration for five times.
- Calculate the molarity of NaOH for five titrations.
- Calculate the RSD for molarity using the formula RSD = Standard Deviation % 100 Mean
- Acceptance criteria: RSD value should not be more than ± 0.5%.
- Enter the details in calibration record as per format-II.
- If the calibration is not within the specified limits repeat the calibration.
- Report the Results to the department head for appropriate action.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-autotitrator-and-electrodes/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Burette calibration record |
| Annexure-II | Auto titrator calibration record |
| Annexure-III | Electrodes calibration record-pH |
| Annexure-IV | Electrodes calibration record-Solvotrode |
| Annexure-V | Electrodes calibration record-Combined Ag ring |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| GTP | : | General Test Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
BURETTE CALIBRATION RECORD
Annexure-II
AUTO TITRATOR CALIBRATION RECORD
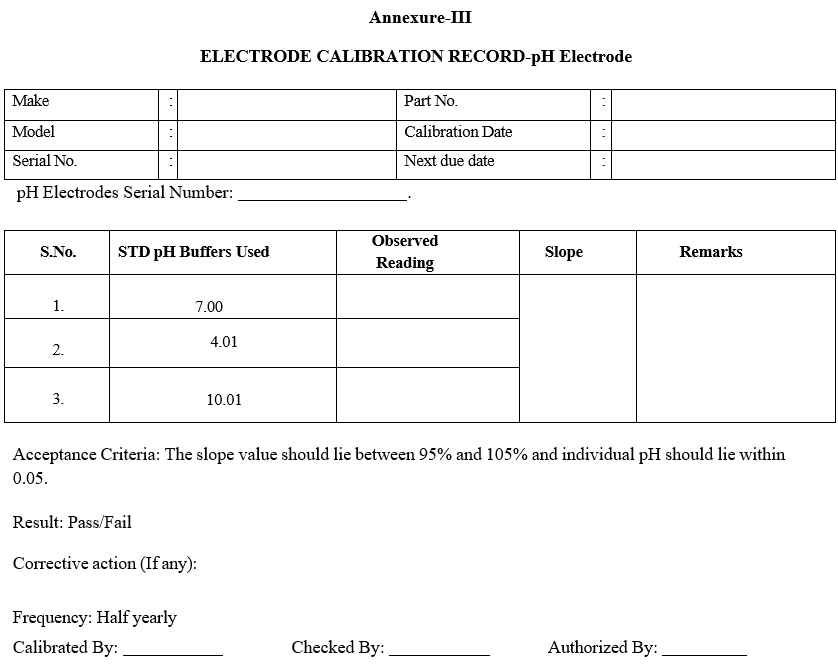
Annexure-III
ELECTRODE CALIBRATION RECORD-pH Electrode
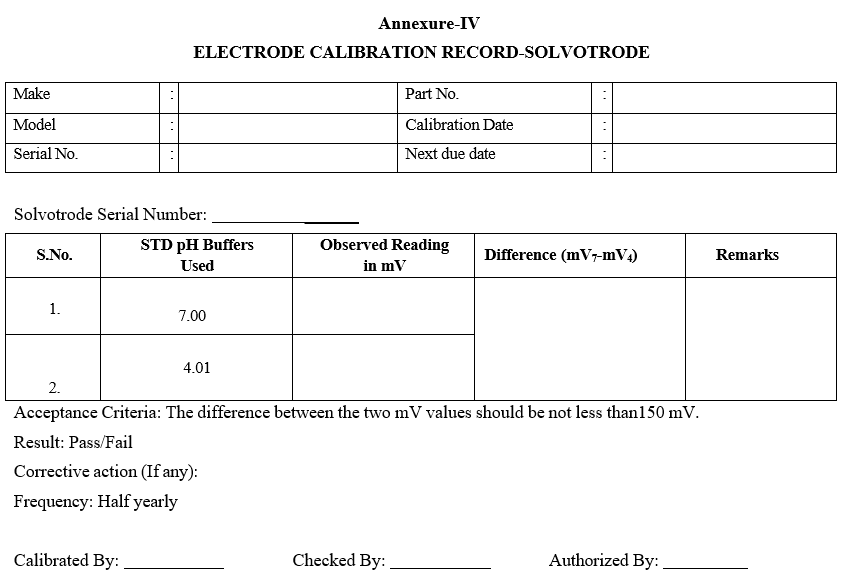
Annexure-IV
ELECTRODE CALIBRATION RECORD-SOLVOTRODE
Annexure-V
ELECTRODE CALIBRATION RECORD-Combined Ag ring
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-autotitrator-and-electrodes/
