- OBJECTIVE:
- To lay down the procedure for Calibration of disintegration test apparatus.
- SCOPE:
This SOP is applicable to the procedure for Calibration of disintegration test apparatus at {Company Name] {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- Head QC: Ensure the compliance of SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
A disintegration test is a quality control procedure used to assess how quickly a solid dosage form, such as a tablet or capsule, breaks down into smaller particles in a liquid medium.
- Ensure that the Instrument and area are clean before calibration.
- Suspend the six cylindrical tubes assembly in the beaker of purified water.
- Adjust the volume of purified water, such that when the assembly is in highest position the wire mesh is at least 15 mm below the surface of purified water and when the assembly is in lowest position the wire mesh is at least 25 mm above the bottom of the beaker and upper ends of the tubes remains above the surface of distilled water.
- Carryout the following tests to calibrate the Disintegration test apparatus.
- Number of dips per minute.
- Temperature.
- Switch ON the Instrument and simultaneously start the stopwatch. The basket rack assembly starts moving up and down. Count the dips per minute.
- Repeat the same for another four times. Note down the number of dips each time.
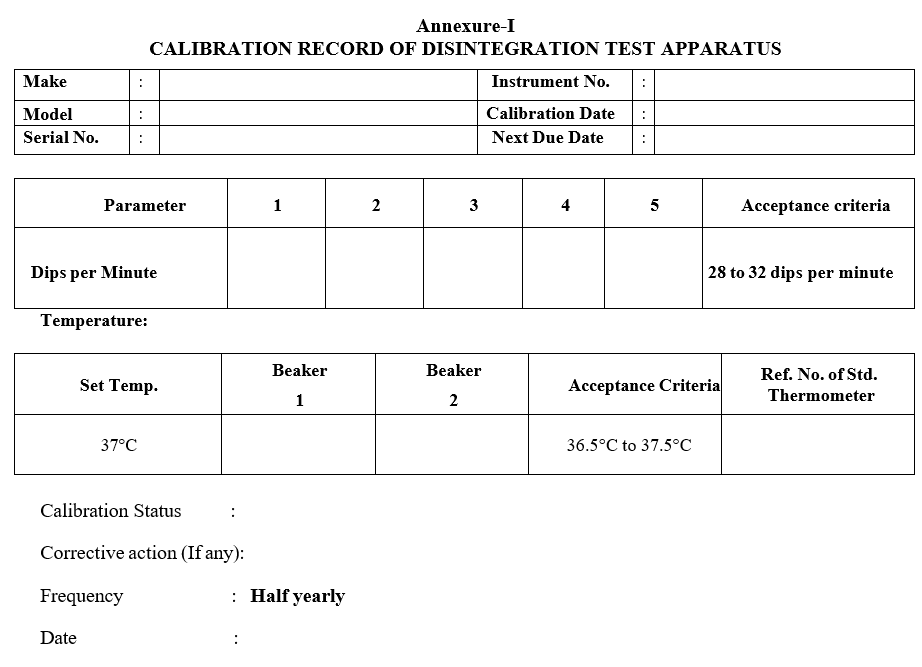
- Record the results in the calibration record as per Format-I.
Acceptance criteria: The number of dips should be in between 28 to 32 in each time.
- Suspend the assembly in a beaker containing distilled water. Adjust the temperature control knob to 37° C. After 30 minutes check the water temperature using a calibrated thermometer. Record the results in the format.
Acceptance criteria: The temperature of water in the beaker should be 37°C ± 0.5°C.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-disintegration-test-apparatus/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Disintegration test apparatus Calibration record |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION RECORD OF DISINTEGRATION TEST APPARATUS
Frequently Asked Question?
Q: What is the purpose of a disintegration test?
A: The purpose of a disintegration test is to assess how quickly a solid dosage form, such as a tablet or capsule, breaks down into smaller particles in a liquid medium.
Q: What are the steps involved in calibrating the disintegration test apparatus?
A: There are 5 steps involved in calibrating the disintegration test apparatus are:
1. Ensure the instrument and area are clean.
2. Suspend the six cylindrical tubes assembly in the beaker of purified water.
3. Adjust the volume of purified water according to the specified criteria.
4. Conduct the dips per minute and temperature tests.
5. Record the results and ensure they meet the acceptance criteria.
Q: What are the acceptance criteria for the number of dips per minute?
A: The acceptance criteria for the number of dips per minute is 28 to 32.
Q: What are the acceptance criteria for the water temperature?
A: The acceptance criteria for the water temperature is 37°C ± 0.5°C.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-disintegration-test-apparatus/
