OBJECTIVE:
To lay down the procedure for Calibration of Dissolution media preparator.
SCOPE:
This SOP is applicable to the procedure for Calibration of Dissolution media preparator at {Company Name} {Location}.
RESPONSIBILITY:
Head QC: Ensure the compliance of SOP.
Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
ABOUT DISSOLUTION MEDIA PREPARATOR:
A Dissolution Media Preparator is a specialized laboratory instrument designed to accurately and efficiently prepare the solutions used in pharmaceutical dissolution testing. This crucial process involves dissolving a specific amount of drug substance in a defined solvent under controlled conditions of temperature and agitation. The preparator ensures precise control over factors like pH, ionic strength, and volume, which are critical for obtaining reproducible and reliable dissolution data. This ensures consistent and accurate results in drug development and quality control, ultimately contributing to the safety and efficacy of medications.
PROCEDURE:
Volume check:
Operate the instrument as per the SOP.
Use the Milli-Q water as media, press proceed and wait until the aspiration completes.
Press aspirate key and enter the amount of 5 Liters to be aspirated, press enter button.
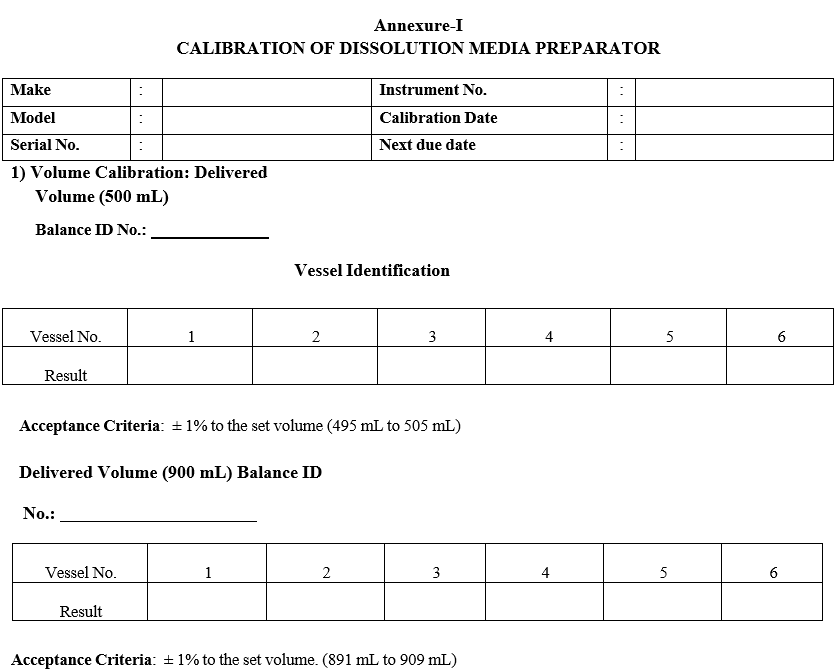
Calibration is recommended for the volumes that will be used (500 mL & 900mL). Set volume as 500ml.
Display indicates to “Put the nozzle into the media container” Note: Performing volume calibration at room temperature (25°C), set temperature control to 25°C and allow the media (water) temperature to stabilize. (Follow as per temperature setting).
Using a calibrated Laboratory Balance, tare with empty vessel, weigh filled vessel, and Record in format-I the actual media weight of the delivered volume for each vessel.
Record in format-I and calculate the media (water) volume for each vessel.
Media volume = Media weight divided by media density at calibration temperature (25°C).
Repeat above 4 steps for the volume 900ml. Note down the readings in Format-I.
Press Dispense key and set the volumes to dispense into the calibrated measuring flask at 900ml and enter the collected volumes in format -I.
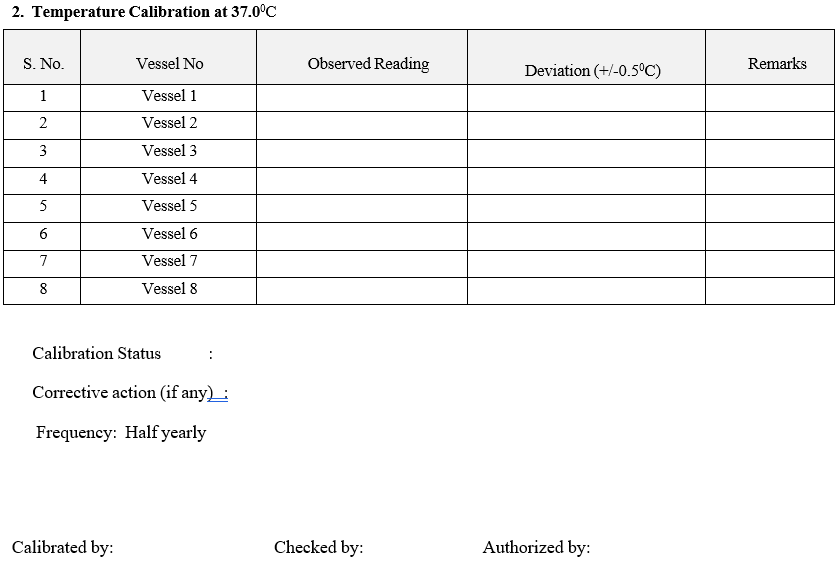
Temperature Check:
Following the above points.
Set the dispense temperature at 37.0 °C. Wait for “Ready to dispense screen”.
Dispense the media into 6 individual test vessels and check the temperature with Calibrated thermometer record the readings in Format-I.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-media-preparator/
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration of Dissolution media preparator |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION OF DISSOLUTION MEDIA PREPARATOR
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-dissolution-media-preparator/

