OBJECTIVE:
To lay down the procedure for calibration of Electronic pipette.
SCOPE:
This SOP is applicable for the procedure for Calibration of Electronic pipette at {Company Name} {Location}.
RESPONSIBILITY:
- Microbiologist: is responsible to perform the activity as per SOP.
- In charge- Microbiology- is responsible to ensure compliance as per SOP.
- Head/Designee Quality Control – Shall be responsible for ensuring compliance as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
About Electronic Pipette:
Electronic micropipettes are precision instruments essential in pharmaceutical research and production. They offer enhanced accuracy and efficiency compared to manual pipettes. These devices feature digital displays for precise volume setting, reducing human error. They often include programmable functions for repetitive tasks, increasing throughput. Additionally, electronic micropipettes can minimize the risk of repetitive strain injuries associated with manual pipetting.
PROCEDURE:
Calibration of Electronic Pipette:
The operating temperature range is not more than 30°C.
Ensure that the thermometer is calibrated.
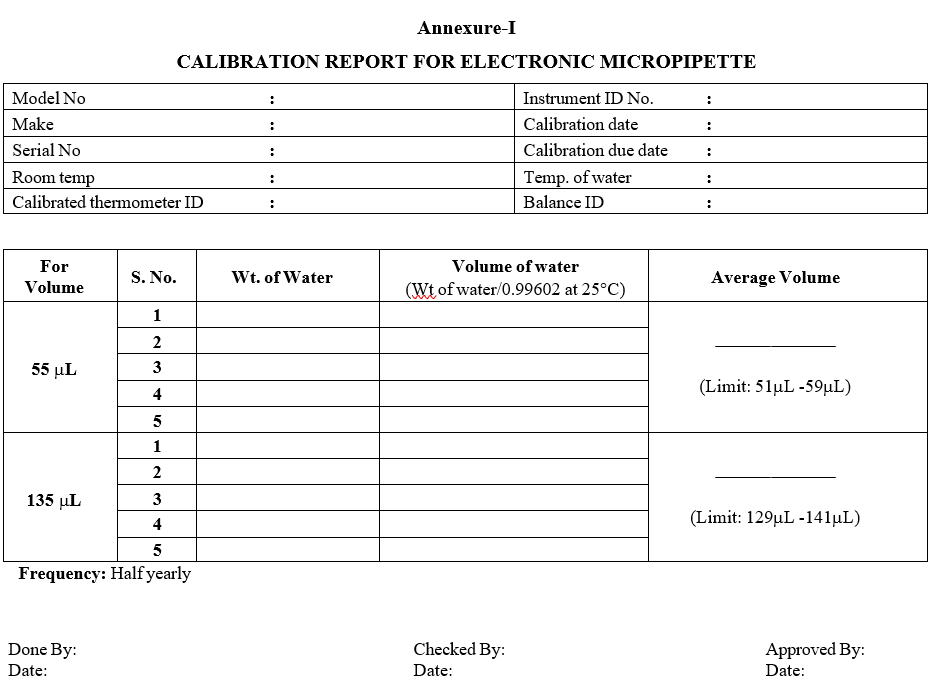
Set the volume of micropipettes having capacity 55 µL and 135 µL.
Calibration shall be carried out between 20°C-30°C.
Ensure that the room temperature shall be NMT 30°C.
ATB Electronic Pipette is an automatic pipette designed to inoculate bacterial and yeast suspensions in ID 32 or ATB TM strips.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/calibration-of-electronic-pipette/
The pipette can inoculate the following volumes:
16 x 135 μL.
32 x 135 μL.
32 x 55 μL.
16 x 55 μL.
Select the parameters 16 x 55μL and 16 x 135 μL as per SOP. Selection of 32 x55 μL and 32 x 135 μL volumes shall also done.
Press the “ ” key to confirm the selected parameter.
Now “ ” appears on the LCD display.
Attach the micro tips to the tip of the Electronic pipette.
Press the “Start” button one time and discharge the water into the appropriate waste bin/beaker.
Press the “start” button for five times and collect the water into the weighed bottle.
Likewise select the parameter for 135 μL and follow the above mentioned procedure.
Divide the average weight of water with 0.99602 to get the required volume and compare it with the permitted volume.
Set volume in Permitted Volume
55µL 51 – 59 µL
135 µL 129 – 141µL
Where ever applicable follow the manufacturer’s instruction manual.
Frequency: Half yearly.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/calibration-of-electronic-pipette/
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration Report for Electronic Micropipette |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control (Micro.)
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| No. | : | Number |
| AC | : | Alternate current |
| LCD | : | Liquid Crystal Display |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION REPORT FOR ELECTRONIC MICROPIPETTE
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/calibration-of-electronic-pipette/


