- OBJECTIVE:
- To lay down the procedure for calibration of Friabilator.
- SCOPE:
This SOP is applicable to the procedure for calibration of Friabilator at {Company Name} {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Operate the instrument as per the SOP.
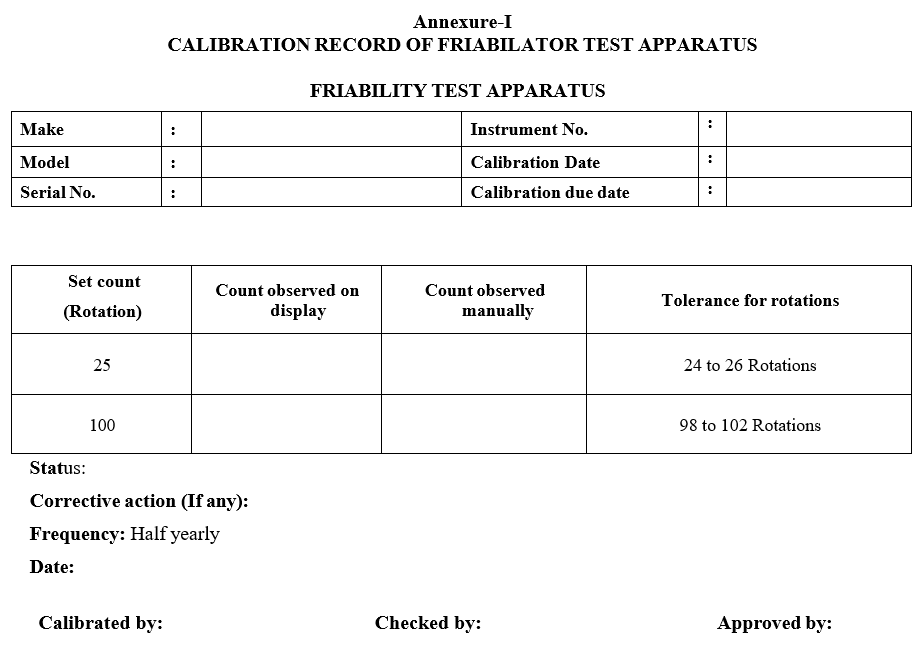
- Set the instrument for 25 rotations and operate.
- Using a stopwatch count the rotations taken for a minute.
- Record the results in the given format.
- The observed rotations should be between 24 to 26 rotations.
- Similarly set the instrument for 100 rotations and operate.
- The observed rotations should be between 98 to 102 rotations.
- Record the results in the given format.
- Calibrate the Instrument Half early or as per internal policy of organization.
- If the results of calibration are not conforming to the specified limits, report to the department head for appropriate action.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-friabilator/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration record of Friabilator Test Apparatus |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| QA | : | Quality Assurance |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION RECORD OF FRIABILATOR TEST APPARATUS
Frequently Asked Question?
1. What is the purpose of operating the instrument according to the SOP?
Answer: To ensure safe, consistent, and reliable operation of the instrument, minimizing errors and maintaining data integrity.
2. What are the two rotation settings used in the procedure, and what are the expected ranges of observed rotations?
Answer: 25 rotations (expected: 24-26) and 100 rotations (expected: 98-102). These ranges indicate acceptable instrument performance based on its specifications.
3. What is the purpose of recording the results in a specific format?
Answer: To maintain organized and easily accessible data for analysis, comparison, and documentation.
4. Why is instrument calibration necessary?
Answer: To ensure the accuracy and precision of the instrument’s measurements over time. Regular calibration compensates for wear, drift, and other factors that can affect measurement accuracy.
5. What happens if the calibration results are outside the specified limits?
Answer: If the observed rotations fall outside the acceptable ranges, it indicates potential instrument malfunction or miscalibration. Reporting to the department head allows for further investigation, potential repair or recalibration, and corrective action to ensure reliable measurements.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-friabilator/

