- OBJECTIVE:
- To lay down the procedure for Calibration of Melting Point Apparatus.
- SCOPE:
This SOP is applicable to the procedure for Calibration of Melting Point Apparatus at {Company Name} {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- Head QC: Ensure the compliance of SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- A melting point apparatus is a scientific instrument used to determine the melting point of a substance. The melting point is the temperature at which a solid changes into a liquid. It is an important physical property of a substance and can be used to identify unknown substances, to test the purity of a substance, and to monitor the progress of a chemical reaction.
- Operate the instrument as per SOP.
- Fill dry powder of Melting Point Reference Standards (USP) in the capillaries individually.
- Insert the capillaries one by one.
- Observe the melting range of each material
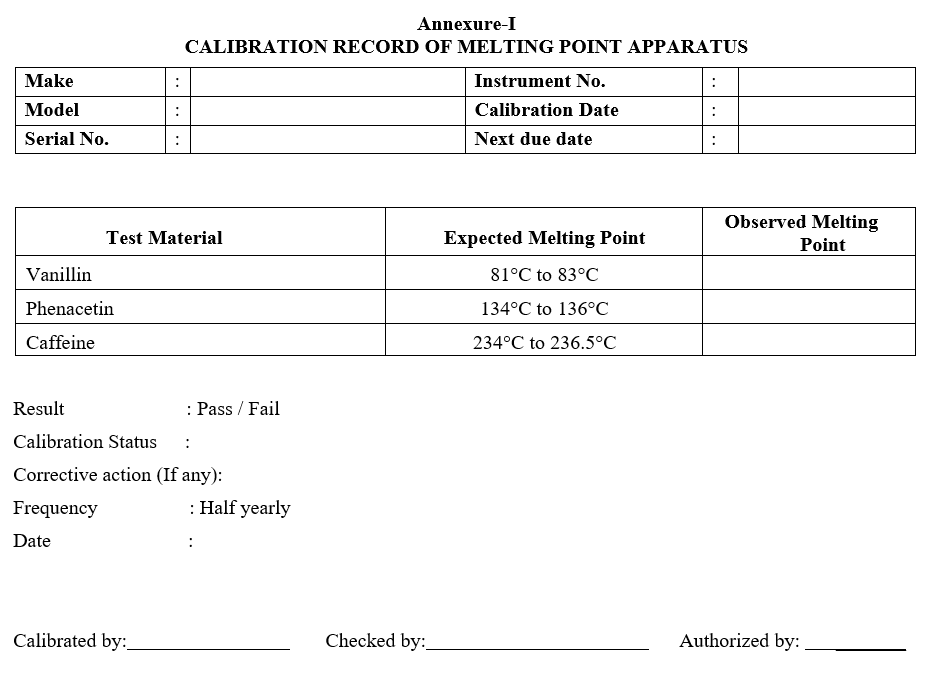
- Melting range should be compared with values given below:
| Test Material | Expected Melting Point |
| Vanillin | 81°C – 83°C |
| Phenacetin | 134°C – 136°C |
| Caffeine | 234° C – 236.5°C |
- Record the results in the format attached (Format-I).
- If the calibration is not proper, repeat the procedure and report the results to the department Head for taking an appropriate action.
- Frequency: Half yearly.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-melting-point-apparatus/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration record of melting point apparatus |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Record Manual |
Annexure-I
CALIBRATION RECORD OF MELTING POINT APPARATUS
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-melting-point-apparatus/
