- PROCEDURE:
- SHIMADZU HPLC WITH EMPOWER SOFTWARE
- MODEL LC-2010CHT
- FLOW ACCURACY TEST:
- Fix the Union/Restriction Capillary in the column compartment.
- Keep degassed water (Milli-Q) in channel ‘A’ and prime the channel thoroughly. Keep the degasser mode on and stabilize for about 1 hour with a flow rate of 1 ml /min.
- Take a 10 ml calibrated volumetric flask and dry it.
- When the flow and pressure are stable, insert the outlet tubing into the volumetric flask and immediately start a calibrated stopwatch.
- Stop the stopwatch when the bottom of the meniscus reaches the 10 ml mark on the flask.
- Record the elapsed time in seconds.
- Calculate the flow rate using the following equation: “Calculated flow rate = (10.00 ml/ measured time in seconds) x 60”.
- Similarly test the flow rate accuracy with flow rates 0.5 mL/min and 2.0 mL/min and record the calculated flow rate
.
- Acceptance criteria: ± 0.02 mL/min of set flow rate.
- Record the results in calibration record as per Format-I.
- Calibration schedule: Once in 6 months and after any major maintenance job.
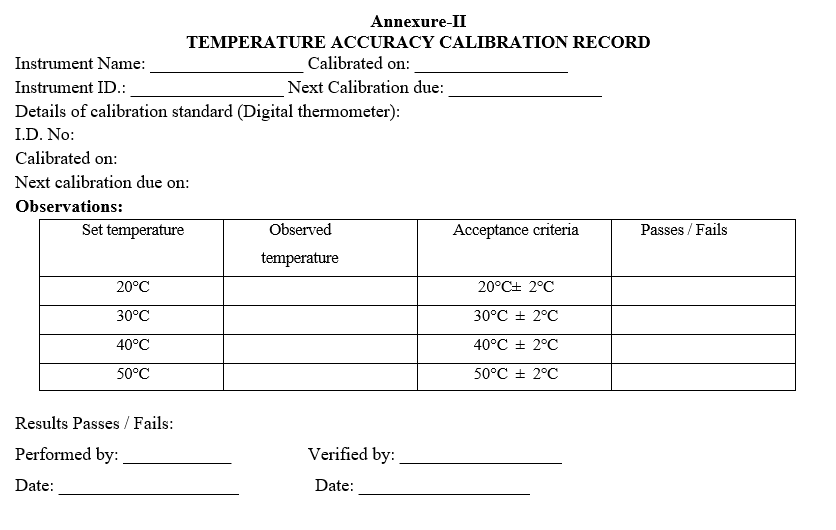
- TEMPERATURE ACCURACY:
- Set column oven temperature to 20ºc and stabilize for about half an hour.
- Keep the temperature probe of Digital thermometer inside the oven compartment and allow to stabilize the reading.
- After stabilization of the temperature, note down the reading as per Format-II.
- Repeat the above 3 steps for the temperatures 30°c, 40°C and 50 °C respectively.
- Acceptance criteria: ± 2ºC of set temperature.
- Record the results in calibration record as per Format – 2 Calibration schedule: Once in 6 months and after any major maintenance job.
- DETECTOR CALIBRATION:
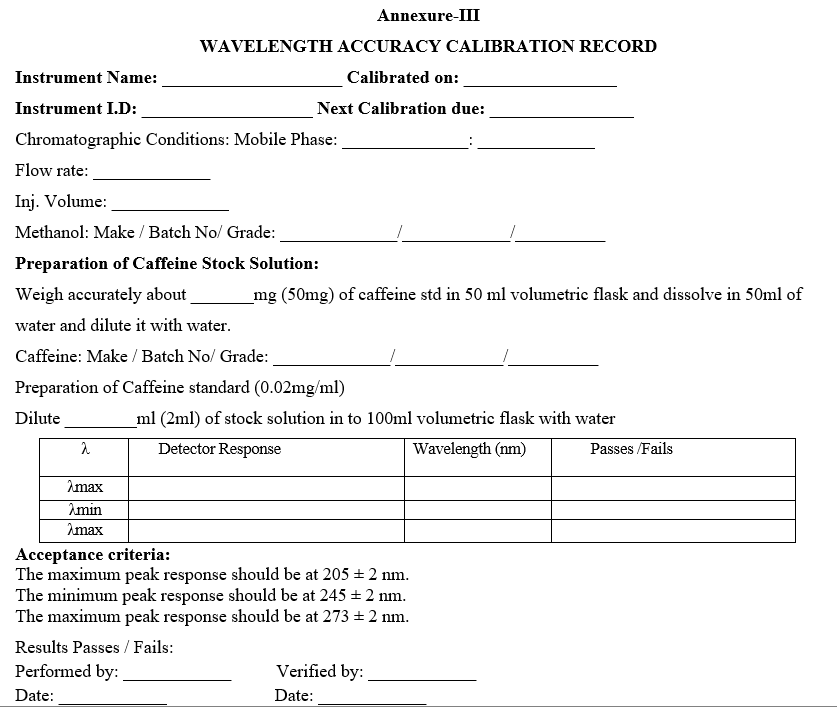
- Wavelength Accuracy
- Operate the instrument as per SOP.
- Weigh accurately 50 mg of caffeine working standard into a 50ml volumetric flask, dissolve and dilute to 50 ml with water. Dilute 2 ml of the above solution to 100 ml with water (0.02 mg/ml).
- Prepare filtered and degassed mobile phase mixture of Water and methanol in the ratio of 70: 30.
- Fix the Column (Symmetry C18, 4.6mm x 75mm 3.5µm) in the column compartment.
- Place the mobile phase and stabilize for 30 minutes at a flow rate of 1.0 ml/min.
- Set the wave length as 201 nm and Column temperature as 35°C.
- Inject the 0.02mg/ml solution each time with the following parameters by varying the wavelength from 201 nm to 209 nm, 241 nm to 249 nm and 269nm to 277 nm with 1 nm frequency.
- Run time 5 min and injection volume 10µl, Perform duplicate injections at all wavelengths.
Acceptance Criteria: The maximum peak area response should be at 205 ± 2 nm. The minimum peak area response should be at 245 ± 2 nm. The maximum peak area response should be at 273 ± 2 nm.
- Enter the results in calibration record as per Format-III Calibration schedule: Once in 6 months and after any major maintenance job.
Note: If the calibration is performed continuously, no further stabilization is required for injector linearity, detector response linearity and wavelength accuracy.
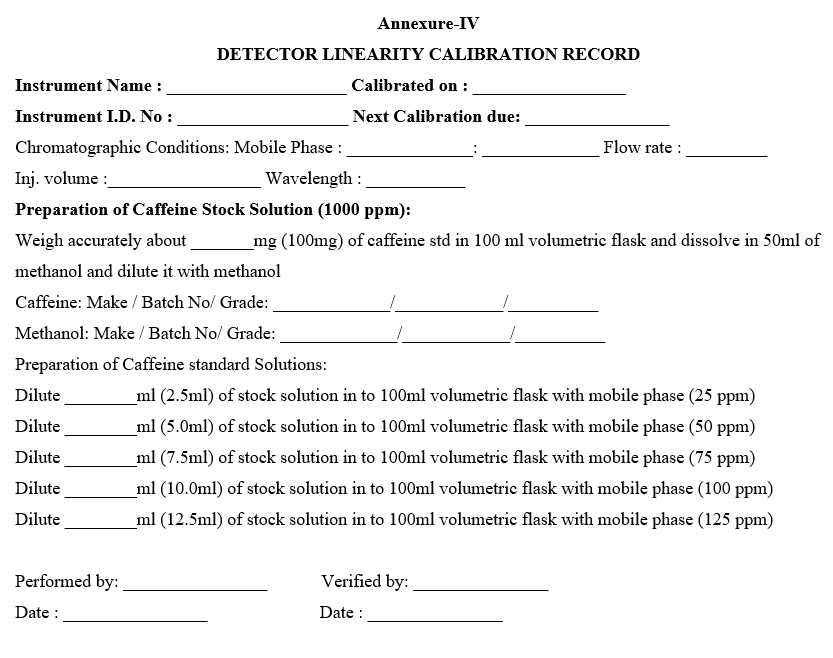
- DETECTOR LINEARITY:
- Fix the Column (Symmetry C18, 4.6mm x 75mm 3.5µm) in the column compartment.
- Prepare the degassed mobile phase mixture of Water and methanol in the ratio of 70: 30, Prepare caffeine working standard solution of 25ppm, 50ppm, 75ppm, 100ppm, and 125ppm concentration from stock solution using mobile phase as diluent.
- Preparation of Caffeine Standard Stock solution (1000ppm): Weigh accurately about 100 mg of caffeine working standard and transfer in to 100 ml volumetric flask dissolve and dilute to volume with methanol.
- Operate the instrument with following chromatographic conditions:
- Wavelength: 274 nm
- Flow rate: 1.0 ml / min
- Injection volume: 10 ml
- Run time: 4 minutes
- Temperature: 25°C
- Preparation of Caffeine Standard solutions:
- Dilute 2.5 ml of stock solution to 100 ml with mobile phase (25ppm)
- Dilute 5.0 ml of stock solution to 100 ml with mobile phase (50ppm)
- Dilute 7.5 ml of stock solution to 100 ml with mobile phase (75ppm)
- Dilute 10.0 ml of stock solution to 100 ml with mobile phase (100ppm)
- Dilute 12.5 ml of stock solution to 100 ml with mobile phase (125ppm)
- Separately inject the above solutions in duplicate and calculate the average peak area response.
- Plot the graph of detector response VS concentration and calculate the detector linearity in terms of correlation coefficient.
- Acceptance criteria: The correlation coefficient should be not less than 0.99.
- Enter the results in calibration record as per Format-IV.
- Calibration schedule: Once in 6 months and after any major maintenance job.
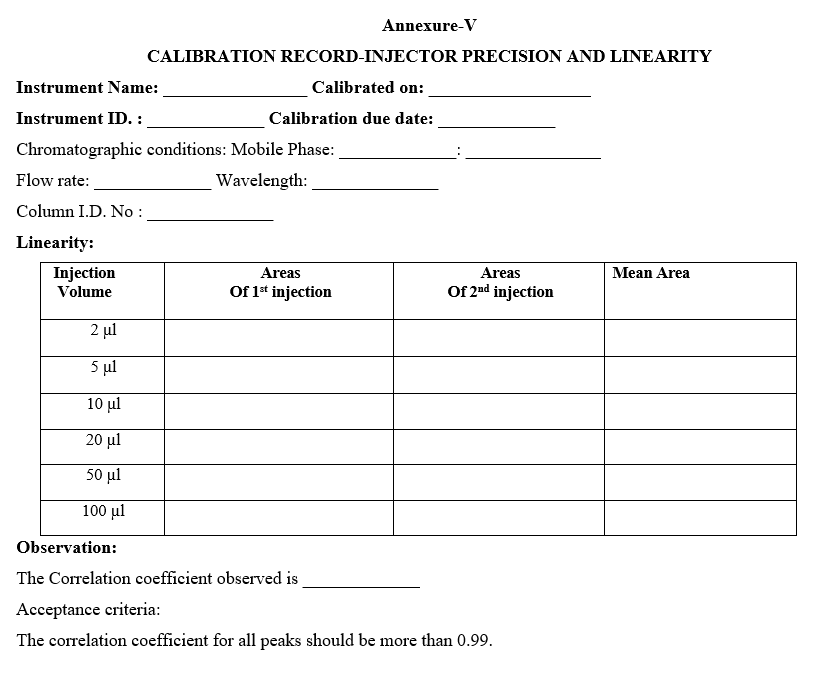
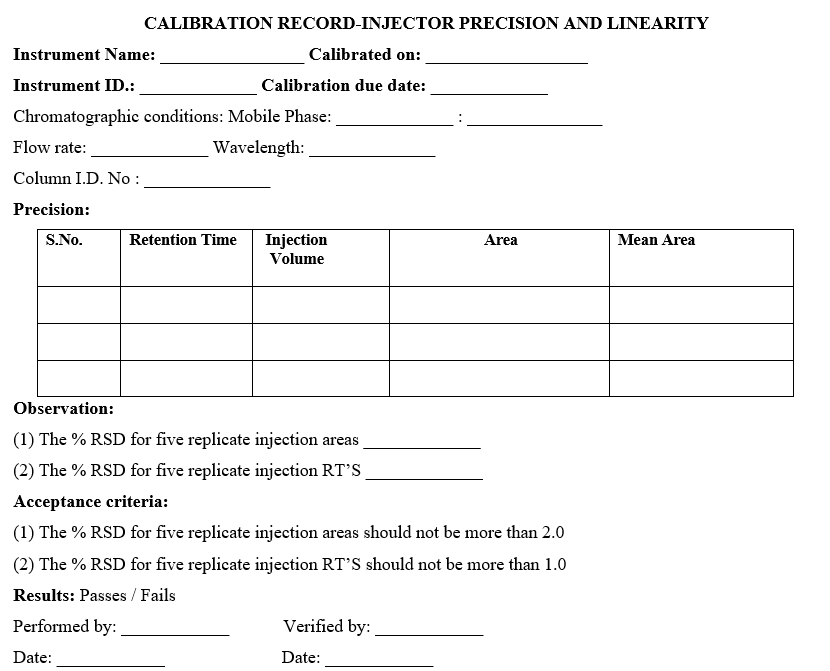
- INJECTOR PRECISION AND LINEARITY TEST:
- PRECISION
- Keep mobile phase (Water and methanol in 70:30 ratio) in channel A stabilize for 10 minutes at a flow rate of 1.0 ml/min.
- Fix the column (symmetry C18, 4.6 mm x 75 mm, 3.5µm) in the column compartment.
- Place the 100ppm caffeine solution sample in carousel.
- Follow the chromatographic conditions for precession
- Wavelength: 274 nm
- Flow rate: 1 ml /min
- Injection volume: 20µl
- Run time: 4 minutes
- Temperature: 25°C
- Inject standard solution five times into the chromatogram and measure the peak area response.
- Acceptance criteria:
The %RSD area of replicate injections is not more than 2.0 %
The %RSD RT of replicate injections is not more than 1.0 %
- LINEARITY:
- Wavelength: 274 nm
- Flow rate: 1 ml /min
- Injection volume
- For 100µl loop: 2µl, 5µl, 10µl, 20µl, 50µl and 100µl
- Run time: 4 minutes
- Temperature: 25°C.
- Separately Inject standard solution with above mentioned injection volume in duplicate and measure the average peak area response.
- Plot the graph of detector response VS injection volume and calculate the injector linearity in terms of correlation coefficient.
- Acceptance criteria: The correlation coefficient should be not less than 0.99.
- Enter the results in calibration record as per Format-V.
Calibration schedule: Once in 6 months and after any major maintenance job.
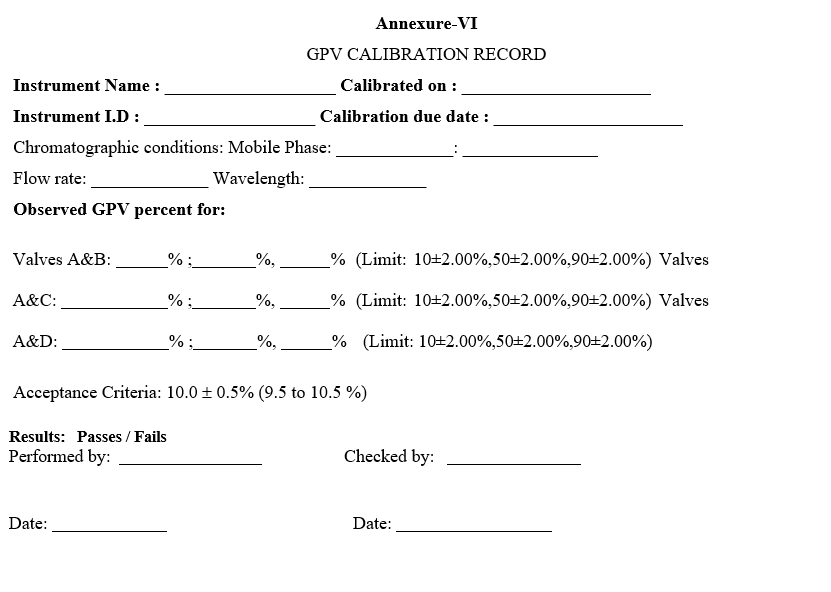
- GRADIENT PROPORTIONING VALVE TEST:
PREPARATION FOR THE ‘GPV’ TEST:
- Connect restrictor tubing (2mm x 0.1 mm ID) in the column compartment.
- Fill the reservoir for the Lines ‘A’ and ‘R’ with Water (100 % water).
- Fill the reservoir for the Lines ‘B’, ‘C’ and ‘D’ with 0.05% Acetone in water.
Note: Degas the purified water before adding Acetone.
- Purge the system with respective solvents for 15 minutes each and stabilize the system for 15 minutes with flow rate of 1.000ml/min. Use GPV-Test instrument method.
- Ensure the Instrument method parameters as follows:
- Wavelength: 254 nm.
- Flow rate : 1.000ml/min.
- Check Gradient Proportion table as follows:
| Time (min) | A. Conc | B-Conc | Curve |
| Initial | 100.0 | 0.0 | 10 |
| 5.00 | 100.0 | 0.0 | 10 |
| 5.01 | 90.0 | 10.0 | 10 |
| 10.00 | 90.0 | 10.0 | 10 |
| 10.01 | 50.0 | 50.0 | 10 |
| 15.00 | 50.0 | 50.0 | 10 |
| 15.01 | 10.0 | 90.0 | 10 |
| 20.00 | 10.0 | 90.0 | 10 |
| 20.01 | 0.0 | 100.0 | 10 |
| 25.00 | 0.0 | 100.0 | 10 |
| 25.01 | 100.0 | 0.0 | 10 |
| 30.00 | 100.0 | 0.0 | 10 |
- Calculate the actual concentration as follows:
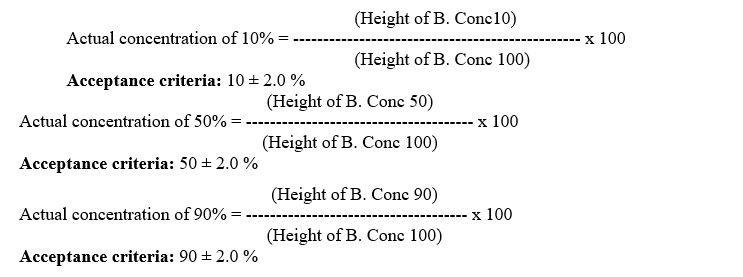
- Verify the concentration accuracy with the solution A/C and A/D in the same way as A/B.
- Record all the results in the calibration record as per Format-VI.
- Report the absorbance values at B, C and D Concentrations of 10%, 50% and 90%.
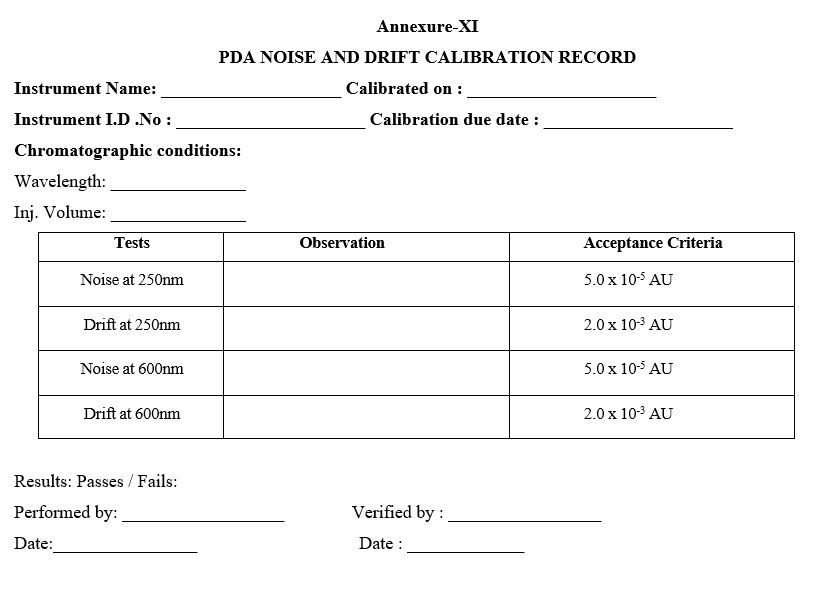
- TEST FOR NOISE AND DRIFT:
- CHROMATOGRAPHIC CONDITIONS:
- Flow rate: 1.000 ml/min.
- Injection Volume: 0 µl
- Run time: 60 min.
- Mobile phase: Water
- Wavelength: 254 nmKeep mobile phase (Water) in channel A stabilize for 60 minutes by connecting a restriction capillary (2mm x 0.1mm ID) in column compartment with a flow rate of 1.000 ml/min.
- Run in single inject window with vial No.1 injection volume 0.0 µl and runtime as 60 min.
- Acceptance criteria: The noise should not be more than 0.125 AU and drift should not be more than 5.0 AU.
- Enter the results in calibration record as per Format-VII.
Calibration schedule: Once in 6 months and after any major maintenance job.
- CALIBRATION OF PDA DETECTOR:
- Wavelength Accuracy:
- In case of PDA detector select the following parameters in instrument method
a) Sampling Frequency: 1.5625 Hz
b) Run Time: 5min
c) Time constant: 0.640 sec
d) Lamp: D2
e) Start wavelength: 190 nm
f) End wavelength: 800 nm
g) Slit Width: 1.2 nm
h) Column temperature: 35°C
- Inject 10µl of 0.02 mg/ml of caffeine in duplicate and after to completion of the run, extract the spectrum from 201 nm to 209 nm, 241 nm to 249 nm and 269nm to 277 nm. Note: For preparation of 0.02mg/ml of caffeine solution.
Acceptance criteria:
The maximum peak area response should be at 205 ± 2 nm.
The minimum peak area response should be at 245 ± 2 nm.
The maximum peak area response should be at 273 ± 2 nm.
- Enter the results in calibration record as per Format-VIII.
Calibration schedule: Once in 6 months and after any major maintenance job.
- PRECISION TEST:
- Keep mobile phase (Water and methanol in 70:30 ratio) in channel A stabilize for 10 minutes at a flow rate of 1.0 ml/min.
- Fix the column (symmetry C18, 4.6 mm x 75 mm, 3.5µm) in the column compartment.
- Place the 10ppm caffeine solution (Dilute 10 ml of 100ppm caffeine standard solution to 100 ml with mobile phase) sample in carousel.
- Follow the chromatographic conditions for precession
- Wavelength: 274 nm
- Flow rate: 1 ml /min
- Injection volume : 10µl
- Run time : 4 minutes
- Temperature: 25°C
- Inject standard solution five times in to the chromatogram and measure the peak area response.
- Acceptance criteria : The %RSD area of replicate injections is not more than 2.0 % The %RSD RT of replicate injections is not more than 1.0 %.
- After completion, test report will be printed. Record the observations as per Format-IX.
- Calibration schedule: Once in 6 months and after any major maintenance job.
- DETECTOR LINEARITY:
- Fix the Column (Symmetry C18, 4.6mm x 75mm 3.5µm) in the column compartment.
- Prepare the degassed mobile phase mixture of Water and methanol in the ratio of 70: 30, Prepare caffeine working standard solution of 25ppm, 50ppm, 75ppm, 100ppm, and 125ppm concentration from stock solution using mobile phase as diluents.
- Preparation of Caffeine Standard Stock solution (1000ppm): Weigh accurately about 100 mg of caffeine working standard and transfer in to 100 ml volumetric flask dissolve and dilute to volume with methanol.
- Operate the instrument with following chromatographic conditions:
- Wavelength: 274 nm
- Flow rate:1.0 ml / min
- Injection volume: 10 ml
- Run time: 4 minutes
- Temperature: 25°C
- Preparation of Caffeine Standard solutions:
a) Dilute 2.5 ml of stock solution to 100 ml with mobile phase (25ppm)
b) Dilute 5.0 ml of stock solution to 100 ml with mobile phase (50ppm)
c) Dilute 7.5 ml of stock solution to 100 ml with mobile phase (75ppm)
d) Dilute 10.0 ml of stock solution to 100 ml with mobile phase (100ppm)
e) Dilute 12.5 ml of stock solution to 100 ml with mobile phase (125ppm)
- Separately inject the above solutions in duplicate and calculate the average peak area response.
- Plot the graph of detector response VS concentration and calculate the detector linearity in terms of correlation coefficient.
- Acceptance criteria: The correlation coefficient should be not less than 0.99.
- Enter the results in calibration record as per Format-X.
Calibration schedule: Once in 6 months and after any major maintenance job.
- TEST FOR NOISE AND DRIFT:
- Connect a resistor tube (2mm x 0.1 mm ID) between the injector outlet piping and the Detector.
- With the flow line filled with degassed water, perform pumping at 1.000mL/min.
- Turn on the detector and set the parameters as follows:
Sampling: 0.640 sec interval
Run Time: 15min
Time constant: 2.00sec
Lamp: D2
Start wavelength: 190nm
End wavelength: 800nm
Injection volume: 0µl
- In Empower Review window, select [Noise and Drift] in the Processing Method, enter the measurement time (15 min) and Segment Width (60sec), and save the Processing Method.
- Set 250nm from ‘Extract Chromatogram’ in the Review window, double-click on the Wavelength marker in the contour plot, and enter 250 nm.
- Double-click on the wavelength marker in the same way and enter 600 nm.
- After selecting ‘Process’ – ‘Integrate’ from the Main menu in the Review window, select ‘Window-Results’ from the Main menu in same way.
- Check the ‘Average Detector Noise (Plot Units) and ‘Detector Drift (Plot Units / hour)’ displayed in the ‘Chromatogram Result’ at the center of the Results window.
- Acceptance criteria: Drift at 250 nm ≤ 2.0 x 10 -3 AU/h Drift at 600 nm ≤ 2.0 x 10-3 AU/h Noise at 250nm ≤ 5.0 x 10-5 AU Noise at 600nm ≤ 5.0 x 10-5 AU
- Enter the results in calibration record as per Format-XI.
Calibration schedule: Once in 6 months and after any major maintenance job.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Flow Accuracy Calibration record |
| Annexure-II | Temperature accuracy calibration record |
| Annexure-III | Wavelength accuracy calibration record |
| Annexure-IV | Detector Linearity calibration record |
| Annexure-V | Linearity and precision calibration record |
| Annexure-VI | Gradient proportioning valve calibration record |
| Annexure-VII | Noise and Drift calibration record |
| Annexure-VIII | PDA Detector Wavelength accuracy calibration record |
| Annexure-IX | PDA Detector Precision calibration record |
| Annexure-X | PDA Detector Linearity calibration record |
| Annexure-XI | PDA Detector Noise and Drift calibration record |
Annexure-I
FLOW ACCURACY CALIBRATION RECORD

Annexure-II
TEMPERATURE ACCURACY CALIBRATION RECORD
Annexure-III
WAVELENGTH ACCURACY CALIBRATION RECORD
Annexure-IV
DETECTOR LINEARITY CALIBRATION RECORD

Annexure-V
CALIBRATION RECORD-INJECTOR PRECISION AND LINEARITY
Annexure-VI
GPV CALIBRATION RECORD
Annexure-VII
NOISE AND DRIFT CALIBRATION RECORD
Annexure-VIII
PDA DETECTOR WAVELENGTH ACCURACY CALIBRATION RECORD
Annexure-IX
CALIBRATION RECORD-PDA PRECISION

Annexure-X
PDA DETECTOR LINEARITY CALIBRATION RECORD


Annexure-XI
PDA NOISE AND DRIFT CALIBRATION RECORD