- OBJECTIVE:
- To lay down the procedure for Calibration of UV- cabinet.
- SCOPE:
This SOP is applicable to the procedure for Calibration of UV- cabinet at {Company Name} {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- Head QC: Ensure the compliance of SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
- Calibration Procedure:
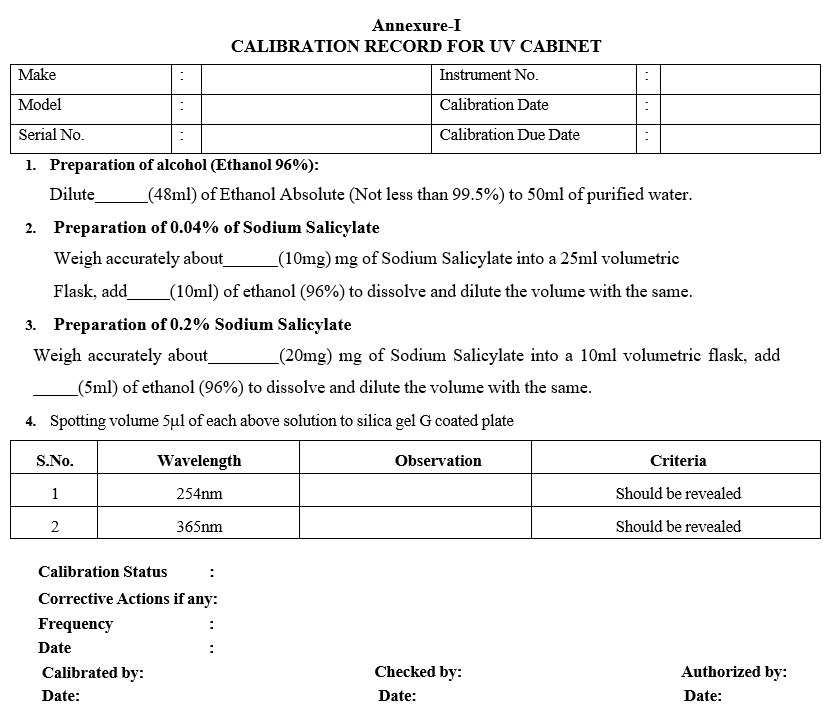
- Preparation of Alcohol (Ethanol 96%): Dilute 48 ml of Ethanol absolute (Not less than 99.5%) to 50 ml with purified water.
- Weigh accurately about 10mg of Sodium salicylate into a 25 ml volumetric flask, add 10 ml Ethanol (96 %) to dissolve and dilute the volume with the same (0.04%).
- Apply 5 ml of the above solution to Silica gel G coated plate.
- Place plate in cabinet and switch on the short wavelength (254 nm) and examine the spot in a position normal to the radiation.
- At the maximum intensity at about 254 nm, the standard spot of Sodium salicylate with a diameter of about 5 mm on chromatographic plate shall be revealed.
- Weigh accurately about 20 mg of Sodium salicylate in 10 ml volumetric flask, add 5 ml of ethanol (96%) to dissolve and dilute the volume with the same (0.2%).
- Apply 5 ml of the above solution to Silica gel G coated plate.
- Place plate in cabinet and switch on the long wavelength (365 nm) and examine the spot in a position normal to the radiation.
- At the maximum intensity at about 365 nm, the standard spot of Sodium salicylate with a diameter of about 5mm on chromatographic plate shall be revealed.
- Enter the results in calibration record as per Format-I.
- Frequency: Quarterly.
- Calibration Procedure:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-uv-cabinet/
- REFERENCES:
British Pharmacopeia
European Pharmacopeia
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration record for UV Cabinet |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| UV | : | Ultraviolet |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION RECORD FOR UV CABINET
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-uv-cabinet/
