- OBJECTIVE:
- To lay down the procedure for Calibration of UV-VIS Spectrophotometer, Make – Shimadzu.
- SCOPE:
This SOP is applicable to the procedure for Calibration of UV-VIS Spectrophotometer at {Company Name} {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- Head QC: Ensure the compliance of SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
A UV-VIS spectrophotometer plays a crucial role in analyzing and characterizing various samples by measuring their interaction with light in the ultraviolet (UV) and visible (VIS) regions of the electromagnetic spectrum.
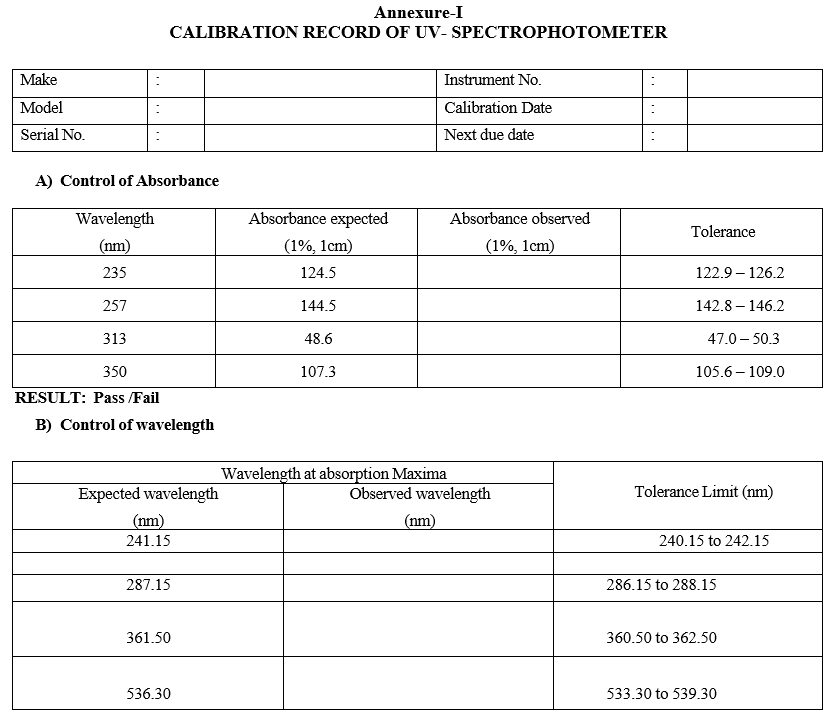
- Control of Absorbance:
- Dry a quantity of Potassium dichromate (AR Grade) by heating to constant weight at 130°C.
- Take 100mg dry Potassium dichromate in 100ml vol. flask and allow to dissolve in 0.01N H2SO4 dilute up to the mark with 0.01N H2SO4 solution.
- Take 6ml of resulting solution dilute to 100ml with 0.01N H2SO4.
- Measure the absorbance of the solution at 235nm, 257nm, 313nm & 350nm taking 0.01N H2SO4 solution as blank.
- Control of wavelengths:
- Prepare the solution of holmium per chlorate as follows.
- Dissolve 0.5 gm of holmium oxide in 2.4 ml of perchloric acid AR by warming gently and diluting to 10ml with purified water.
- Check the extinction of the solution, permitted tolerance is 1nm for the range 200 to 400 nm and 3 nm for the range 400 to 600 nm.
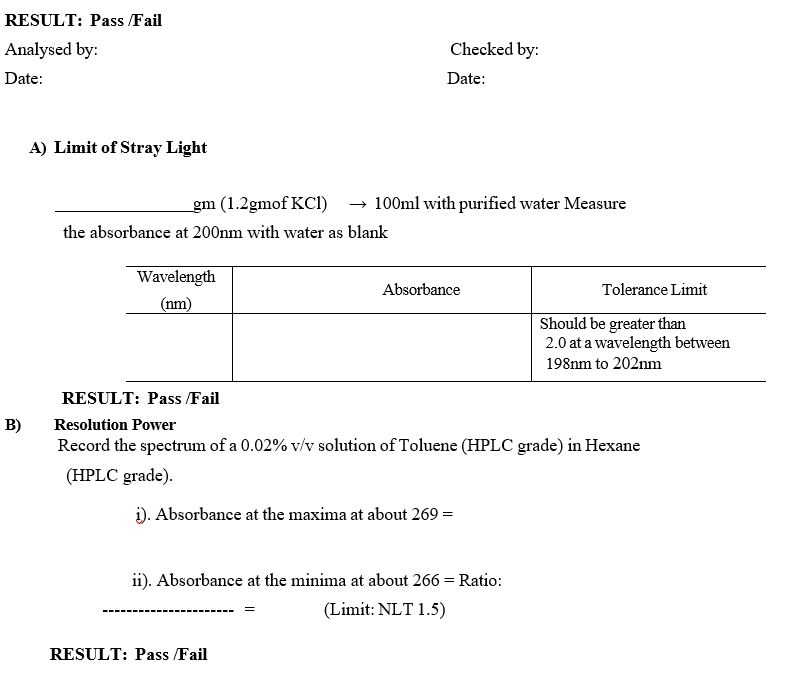
- Limit of Stray Light:
- Prepare 1.2% w/v solution of Potassium Chloride.
- Measure the absorbance at 200nm.
- Absorbance should be not less than 2.0.
- Resolution Power:
- Prepare a 0.02% v/v solution of Toluene (HPLC grade) in Hexane (HPLC grade).
- Record the ratio of the absorbance at the maxima at about 269 nm to that at the minima at about 266 nm.
- Ratio should not be not less than 1.5.
- Report the result in the format.
- Frequency: Half early.
- Action: If the calibration is not proper then repeat the procedure and report the results to the department head for an appropriate action.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-uv-visible-spectrophotometer/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Calibration record of UV- spectrophotometer |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| UV | : | Ultraviolet |
| KOH | : | Potassium Hydroxide |
| HPLC | : | High Performance Liquid Chromatography |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
CALIBRATION RECORD OF UV- SPECTROPHOTOMETER

Frequently Asked Question ?
Q: Why is Potassium Dichromate used for absorbance control?
A: Potassium Dichromate is a certified reference material with well-defined absorption peaks at specific wavelengths. Measuring its absorbance at these wavelengths allows us to verify the accuracy of the spectrophotometer’s absorbance readings.
Q: What does “drying potassium dichromate to constant weight” mean?
A: Heating the potassium dichromate removes any moisture it may have absorbed, ensuring the prepared solution has an accurate concentration. Constant weight implies no further weight change with continued heating.
Q: Why are specific dilutions prepared ?
A: The final solution needs to be within a suitable absorbance range for reliable measurement. Diluting a concentrated solution allows achieving this desired range.
Q: What is the purpose of using Holmium Perchlorate for wavelength control?
A: Holmium Perchlorate has sharp emission lines at known wavelengths. By comparing the measured peak positions with the reference values, we can verify the accuracy of the instrument’s wavelength readings.
Q: What does “limit of stray light” refer to and why is it important?
A: Stray light is unwanted light entering the detector besides the main beam passing through the sample. High stray light can lead to inaccurate absorbance readings. Measuring the absorbance of a highly absorbing solution at a wavelength where it should be opaque (like Potassium Chloride at 200nm) helps assess stray light levels.
Q: What does “resolution power” signify in this context?
A: Resolution power reflects the instrument’s ability to distinguish closely spaced peaks. Measuring the absorbance ratio of Toluene in Hexane at specific wavelengths helps evaluate this capability.
Q: How often should calibration be performed?
A: The paragraph suggests “half early” based on internal policy, possibly meaning half the interval between scheduled calibrations. Regular calibration ensures reliable measurements over time.
Q: What happens if the calibration fails?
A: If the results fall outside specified limits, repeating the procedure is recommended. If issues persist, reporting to the department head is crucial for further investigation and potential instrument repair or adjustments.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-uv-visible-spectrophotometer/
