- OBJECTIVE:
- To lay down the procedure for Calibration of Vacuum Oven, Make: Newtronic.
- SCOPE:
This SOP is applicable to the procedure for Calibration of Vacuum Oven at {Company Name} {Location}.
- RESPONSIBILITY:
- Executive/Designee Quality Control is responsible to calibrate the instrument as per SOP.
- Head QC: Ensure the compliance of SOP.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE:
A vacuum oven is a specialized oven that removes moisture and other gases from a substance by creating a low-pressure environment inside the chamber. This is different from a standard oven, which uses heat and air circulation to dry materials.
- Operate the instrument as per SOP.
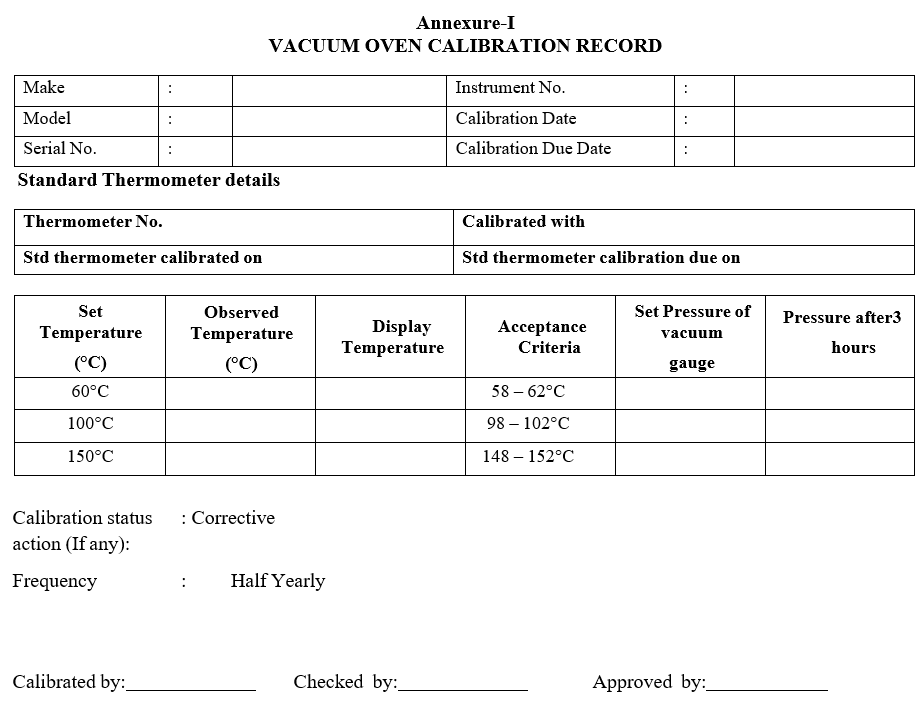
- Set the temperature controller knob to 60°C.
- After the set temperature is attained, measure it with a calibrated thermometer.
- The observed temperature should be within ± 2.0°C.
- Similarly perform the test at 100°C and 150°C and measure the temperature with a calibrated thermometer.
- The observed temperature should be within ± 2.0°C.
- Record the readings in vacuum oven calibration record as per Format-I.
- Create a vacuum of 400 mm and close the valve and observe the Vacuum drop for 3 hours. The Vacuum drop should be not more than 1.0% of the initial Pressure after 3 hours.
- The frequency for calibration of vacuum oven is Six months.
- If the calibration is not proper, report the results to the departmental head for taking an appropriate action.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-vacuum-oven-make-newtronic/
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Vacuum Oven Calibration record |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Quality Control
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
VACUUM OVEN CALIBRATION RECORD
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/calibration-of-vacuum-oven-make-newtronic/
