OBJECTIVE:
To lay down the procedure for Calibration of Meshes.
SCOPE:
This SOP is applicable for storage of Calibration of Meshes at {Company Name} {Location}.
RESPONSIBILITY:
- Initiator Officer/Designee: Production shall perform the operation activity as per SOP.
- Initiator Executive/Designee: Production shall ensure the compliance of the SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
Procedure for calibration of meshes:
Check the “CLEANED” label of mesh before starting the calibration.
Take the plain paper and mark with pen one linear square inch in the paper with the help of calibrated standard measuring scale.
Keep the marked paper below the mesh and count the apertures with the help of magnifying glass.
Count the number of holes in linear inch in both directions (Horizontal and Vertical direction).
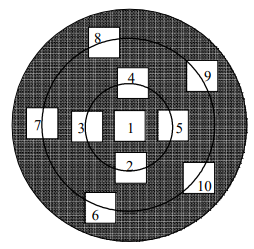
Take minimum ten location counts in each mesh.
Refer the flow chart for the location.
Calibrate the mesh count below or equal to 30# following by the above said procedure.
Calibrate the mesh count more than 30# using Densimeter as mentioned below.
Calibration Of Meshes Using Densimetre:
- Keep densimeter on the sieve in such way letters are facing us and other side is touching the sieve.
- Move the densimeter so that any one vertical line of densimeter is matching with the vertical line of the mesh.
- Grids will start forming on the densimeter. Peak of grid form like the peak of the mountain.
- The line of this peak where it matches the mesh number mentioned on the densimeter is the actual mesh number of the sieve.
The tolerance limit is as mentioned in the table for ‘Tolerance limit’.
All the meshes calibrate once in a six months and records to be maintained as per Format-1.
If any discrepancy observed immediately inform to the concerned production in charge and Department head for the necessary action.
TOLERANCE LIMIT
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/calibration-procedure-for-meshes/
| Sl. No. | Mesh size | Number of apertures per linear inch |
| 1. | 10# | 9-11 |
| 2. | 12# | 11- 13 |
| 3. | 14# | 13 -15 |
| 4. | 16# | 15 – 17 |
| 5. | 18# | 17 – 19 |
| 6. | 20# | 19- 21 |
| 7. | 24# | 23 – 25 |
| 8. | 25# | 24 – 26 |
| 9. | 30# | 29 – 32 |
| 10. | 35# | 33-37 |
| 11. | 40# | 38 – 42 |
| 12. | 50# | 48 – 52 |
| 13. | 60# | 57 – 63 |
| 14. | 80# | 76 – 84 |
| 15. | 100# | 95 -105 |
- If any new meshes are received need to be calibrated before issue to the production.
- Damaged sifter sieve should be sent for rescreening as per SOP.
- Rescreened sieve should be numbered as per SOP “Procedure for coding of screens, meshes, FBP bags and bellows.
- If any sieve fails during calibration then PNC shall be raised and the sieve shall be subjected for rescreening.
REFERENCES:
Not Applicable
- ANNEXURES:
| Annexure no. | Title of annexure |
| Annexure-I | Mesh calibration Log Sheet |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
Master Copy : Quality Assurance Department
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head Production
ABBREVIATIONS:
| PD | : | Production |
| MNC | : | Material Non-Conformance |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| UOM | : | Unit Of Measurement |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/calibration-procedure-for-meshes/