OBJECTIVE:
To lay down the procedure for cleaning and sanitization of Pharmaceutical Industry.
SCOPE:
This SOP is applicable to the procedure for cleaning and sanitization of Pharmaceutical Industry at IM Healthcare Pvt. Ltd. Baddi.
RESPONSIBILITY:
- HR Executive/Designee – is responsible to follow the procedure as per SOP.
- Housekeeping: is responsible for cleaning of toilets.
- Head HR – is responsible for compliance of the SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
Importance of Cleaning and Sanitization in Pharmaceuticals:
Cleaning and sanitation in the pharmaceutical industry is a critical aspect of ensuring product safety and quality. Stringent cleaning and sanitation protocols are implemented to eliminate contaminants like bacteria, viruses, and other microorganisms that could compromise the integrity of medications. These procedures involve meticulous cleaning of surfaces, equipment, and production areas, followed by thorough disinfection using approved sanitizing agents. Regular monitoring and validation of cleaning and sanitation processes are essential to maintain compliance with regulatory standards and ensure the production of safe and effective pharmaceutical products.
PROCEDURE:
Areas:
Process Areas: Granulation area, Compression area, coating areas, Blending area, inspection areas, Dispensing area.
Non-process areas: Change rooms, corridors, office room, Tablet hold areas, Quarantine area, Washroom, Airlocks, RM store.
Quality Control Lab: Instrument room, dissolution room, wet lab, hot room, qc staff room, glassware washing room, audit room, qc manager room, passage, corridors & sample checking.
Visitors Entry areas: Visitor entry and exit room.
General Areas: Steps, Corridors, Water system, service floor, outer corridors, roads, linen room, Laundry.
Cleaning Tools & Equipment:
- For Dusting and wiping all fixtures – Lint free dusters.
- For mopping the floor – Lint free Mop.
- For glass cleaning – Lint free Glass cleaning duster.
- For scrubbing the doors – Nylon scrubber.
- For cleaning the Return air duct grills – Soft Nylon brush/ Lint free duster.
- For cleaning the stains on the walls –Lint free cloths.
- For collecting the dust from floor surfaces – dustpan with soft nylon brush.
- For removing the excess water by pushing it towards the drain outlet –Squeeze.
- For cleaning the door window panels – spray can with glass cleaner & Squeeze.
- For floor mopping-double bucket mopping trolley with micro fibre lint free mop refills for cleaning the areas.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/cleaning-and-sanitization-in-pharmaceutical-industry/
- For cleaning the walls- super mop.
- For cleaning the wall, stainless steel wall cleaning rod – Allklean, windows shine.
- For Floor dry cleaning all surfaces- dry mop.
Disinfectants- The frequency of changing the disinfectant for cleaning in Process areas.
Disinfectant solutions prepare required dilutions, frequency daily.
| S No. | Dates | Name of the Disinfectant | Concen tration | Dilution (Disinfectant Solution Qty) | Purified water Qty. | Prepared disinfectant solution Qty. | Remarks |
| 1 | 1st Week | Bacillocid special -0.5% v/v | 0.5% | 50 Ml | 9.950 Ltr. | 10 Ltr. | For process and non- process areas |
| 2 | 2nd Week | Sokrena -2% v/v | 2% | 200 Ml | 9.800 Ltr. | 10 Ltr. | |
| 3 | 3rd Week | Korsolex 2% v/v | 2% | 200 Ml | 9.800 Ltr. | 10 Ltr. | |
| 4 | 4th Week | Bacillocid special -0.5% v/v | 0.5% | 50 Ml | 9.950 Ltr. | 10 Ltr. | |
| 5 | 5th Week | Sokrena -2% v/v | 2% | 200 Ml | 9.800 Ltr. | 10 Ltr. |
Cleaning agents for General areas:
General disinfectants & Detergent solutions prepare required dilutions frequency daily.
| S No. | Dates | Name of the general Disinfectant & Cleaning agents. | Concentration | Dilution (Disinfectant) Solution Qty.) | Purified water Qty. | Prepared disinfectant solution Qty. | Remarks |
| 1 | 1st Week | Lizol | 2% | 200 Ml | 9.800Ltr | 10 Ltr. | General areas |
| 2 | 2nd Week | Dettol | 2% | 200 Ml | 9.800 Ltr | 10 Ltr. | General areas |
| 3 | 3rd Week | Lizol | 2% | 200 Ml | 9.800Ltr | 10 Ltr. | General areas |
| 4 | 4th Week | Dettol | 2% | 200 Ml | 9.800 Ltr | 10 Ltr. | General areas |
| 5 | 5th Week | Lizol | 2% | 200 Ml | 9.800Ltr | 10 Ltr. | General areas |
| 6 | General | Allklean | 1% | 100 Ml | 9.900 Ltr | 10 Ltr. | Multi purpose |
| 7 | General | Teepol | 2% | 200 Ml, | 9.800 Ltr | 10 Ltr. | Detergent |
| 8 | General | Window shine | Ready to use | – | – | Glass cleaning | |
Floor cleaning:
Dry mop the floor in all areas & rooms.
Clean the floor with the cleaning agent/diluted disinfectant solution using the floor scrubber drier machines for scrubbing and moping the floor or double bucket trolley with diluted disinfectant solution for moping.
Frequency: Daily.
Clean the floors, doors, walls, Ceiling /A.C grills and coving portion with lint free mop dampened in disinfectant solution, leave the area up to 10 minutes after disinfectant application.
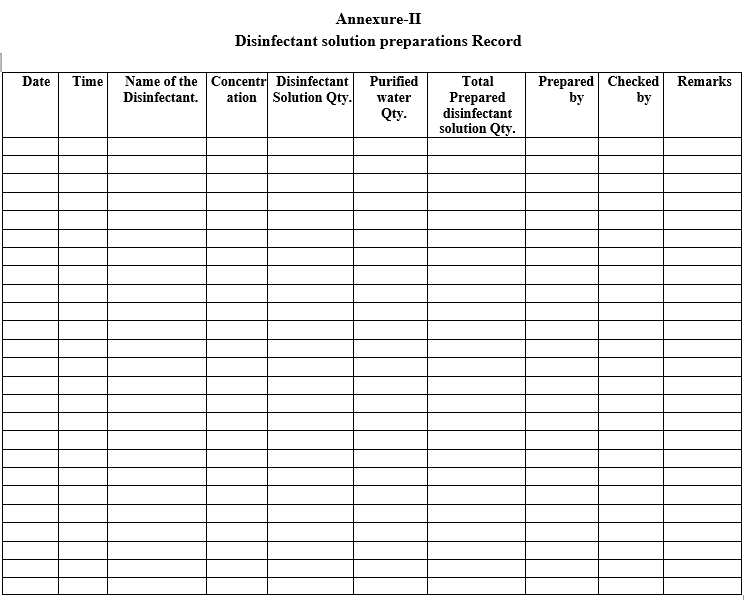
For preparation of disinfectant solution purified water shall be collected from FG & PM store wash room, and same was kept into the housekeeping janitor room for preparation of disinfectant solution.
Record the details of disinfectant solution preparation as per the Format-III.
Waste bin cleaning:
Remove polythene bag kept in the waste bin, tie the bag with a nylon string and kept it ready for collection at collection point.
Clean the waste bin and place the new Polybags.
Frequency: Twice in a day as and when required.
General areas and process areas daily Cleaning:
Clean the doors, wall fittings; windows, switch boards, cables in all the rooms, Dress cabinets, hand disinfectant container and, its holder gloves, entry & Exit photos, soiled garments bin and waste bin in Gowning room.
Clean the supply and return air grills and tube light fittings (along with the surface). Using dry mop\soft Nylon brush\ lint free cloth. Frequency of cleaning once in a week.
Clean the walls and ceiling with lint free mop dampened in disinfectant solution. Frequency of cleaning once in a week.
Take a clean micro fiber mop or Roller sponge (Super mop) rod and clean the entire ceiling & walls with the cleaning agent followed by diluted disinfectant solution.
While using disinfectant solution dip the roller sponge (Super mop) /mop into it and Squeeze the extra solution before cleaning the walls.
Start giving even smooth strokes starting from the ceiling to the floor.
Ensure that every stroke overlaps the other.
Remove wall marks if any by spotting with clean sponge and cleaning agent immediately as and when required.Vacuum the Return air raiser grill from inside once it is removed by maintenance department for cleaning,
Clean inside Return air raiser duct and grill with cleaning agent followed by disinfectant solution.
Take a wet cloth and wipe the doors including handles and door closers.
Wipe the window and door panels with a dry mop / duster and wipe it clean by using glass cleaner without leaving any streaks.
Wipe the waste bins from outside and inside using a dry duster followed by wiping with clean wet duster.
Clear the drains Remove the cover and keep it aside, Withdraw the drain catch pit container.
Clear all accumulated debris and clean the drain with sufficient quantity of water.
Clean the entire floor with Floor scrubber drier followed by mopping with diluted disinfectant solution.
Clean the cob webs using telescopic cub web Nylon brush in outside and general areas.
Clean all the cleaning equipments, tools.
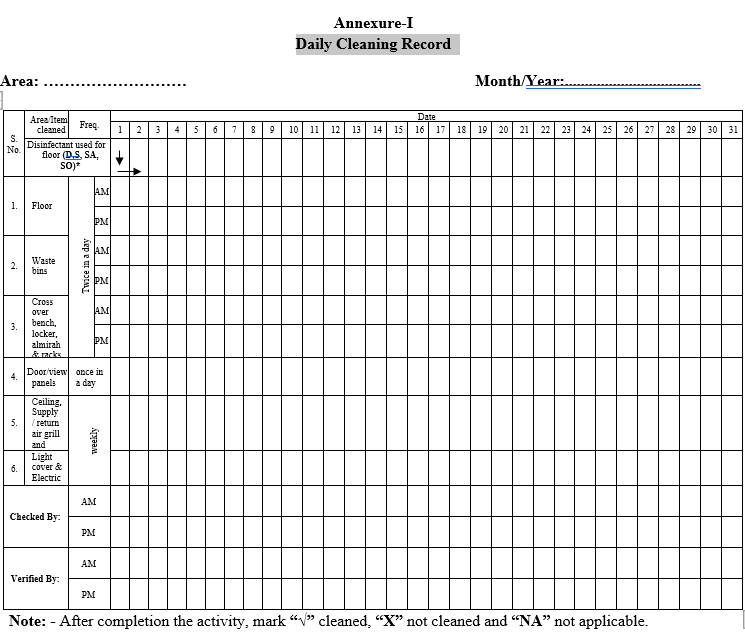
Record the cleaning operation as per the Format-I.
Records shall be checked by supervisor House Keeping and verified by Executive / area in charge.
Frequency: Daily, when required.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/cleaning-and-sanitization-in-pharmaceutical-industry/
REFERENCES:
Not Applicable
ANNEXURES:
| Annexure No | Title of annexure |
| Annexure-I | Daily Cleaning Record |
| Annexure-II | Disinfectant solution preparations Record |
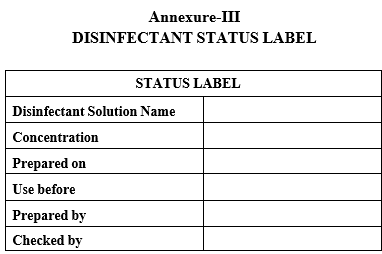
| Annexure-III | Disinfectant Status label |
ENCLOSURES: SOP Training Record.
DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Human Resources
- Master Copy : Quality Assurance Department
ABBREVIATIONS:
| HR | : | Human resources |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| IPA | : | Iso Propyl Alcohol. |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Annexure-I
Daily Cleaning Record
Annexure-II
Disinfectant solution preparations Record
Annexure-III
DISINFECTANT STATUS LABEL
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/cleaning-and-sanitization-in-pharmaceutical-industry/


