- OBJECTIVE:
To describe a standard operating procedure for collection, storage and disposal of hazardous waste.
- SCOPE:
This Procedure is applicable for collection, storage and disposal of hazardous waste at {Company Name} {Company Location}.
- RESPONSIBILITY:
- In charge- User department
- In charge – EHS
- ACCOUNTABILITY:
QA Manager shall be accountable for Approval & Implementation of SOP.
- PROCEDURE:
Definition: Waste that poses a risk to human health or the environment and requires special disposal techniques to make it harmless or less dangerous.
- Hazardous waste is categorized into the following types:
- Daily sweepings of production areas.
- Remaining samples at QC Off Specification raw materials Off Specification Finished Products Expired raw materials.
- Expired Finished products. Rejected products.
- Stability samples QC expired and discarded samples.
- Any other hazardous wasteMicrobiology culture waste
- Collection and storage of Hazardous waste:
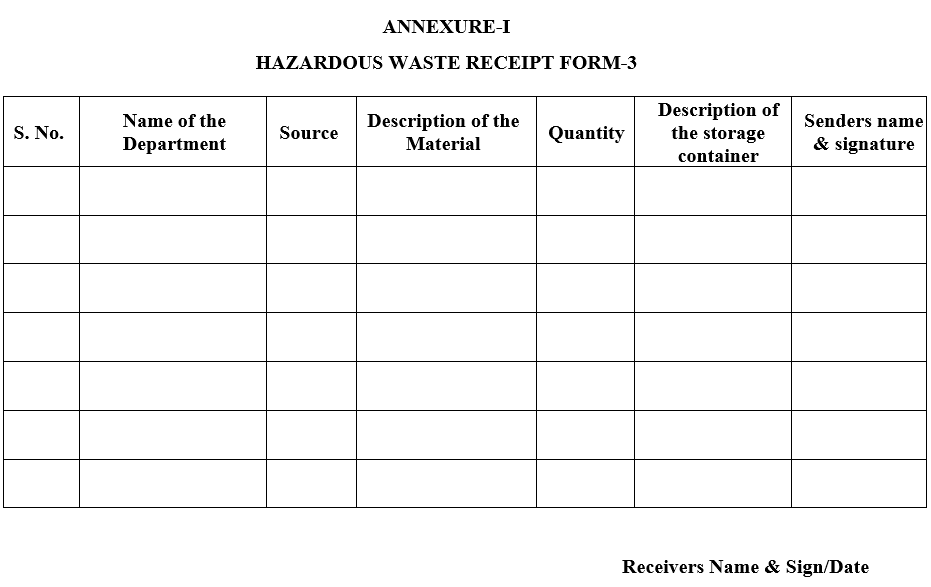
- At the time of collecting hazardous waste, Form -3 (Hazardous waste Receipt) is filled as per Annexure-I.
- Preparation and Usage of 4 % Sodium Hypochlorite solution as per Annexure-II.
- Waste shall be collected after necessary deactivation (deactivation done by using 4% Sodium Hypochlorite solution for 48 Hrs.) and by concerned, wherever necessary and handover to the SHE department.
- Waste not requiring deactivation shall be collected and transferred to the hazardous waste’s storage and handling area.
- Wastes received after deactivation are in slurry form, such wastes shall be filtered, and the residue shall be collected and stored. The filtrate shall be transferred to wastewater collection and pre-treatment.
- Personnel protective Equipment must be used while collection, transfer of all Hazardous waste.
- Disposal of Hazardous waste:
- Wastes shall be milled/defaced as per requirement based on waste received.
- Wastes shall be disposed after one full truckload / full container load is accumulated.
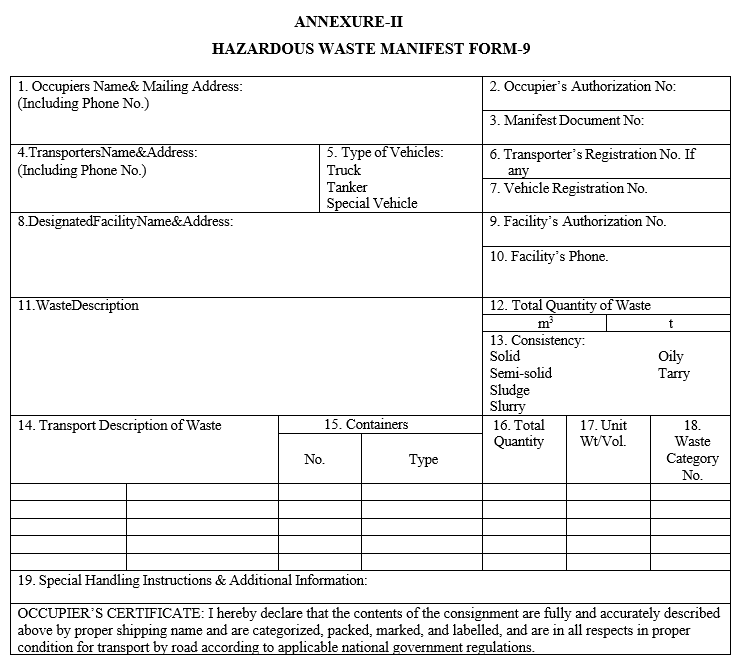
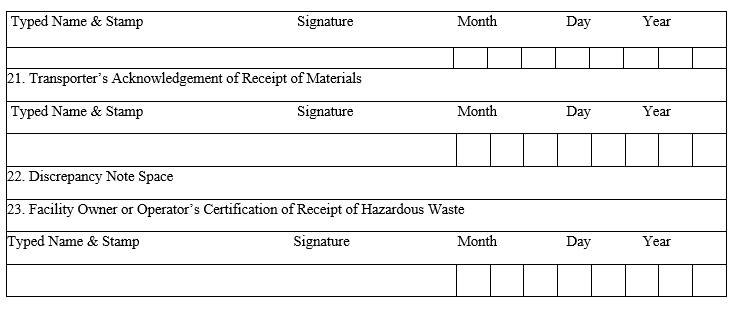
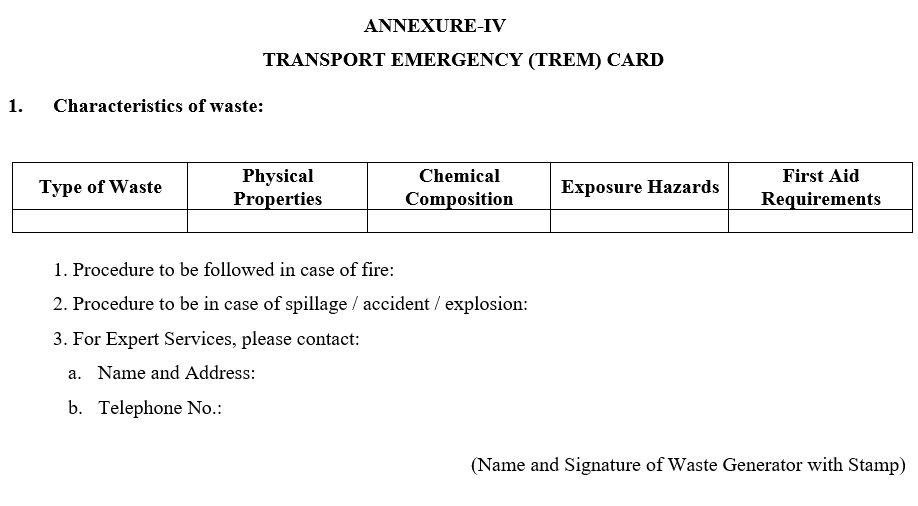
- Wastes shall be disposed to cement units/TSDF along with Form – 9 (Hazardous Waste Manifest) as per Annexure-II and Form – 10 (Transport Emergency (TREM) Card).
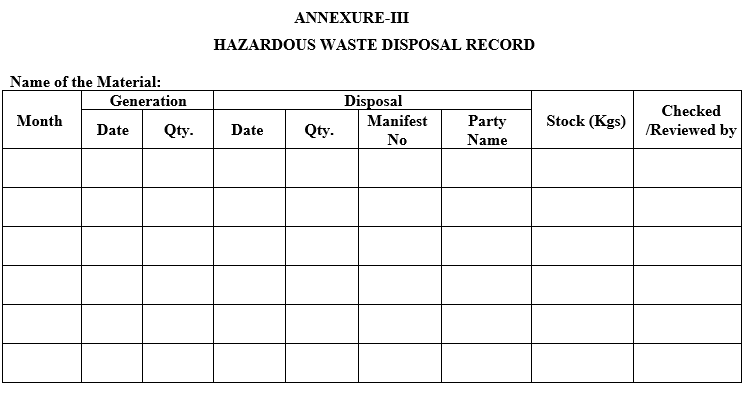
- Record the received and disposed quantities in ‘Hazardous waste disposal Record’ Annexure-III
- File all the Manifests including Acknowledged copies.
- Disposal of Hazardous waste:
- Microbiology culture waste shall be disposed to Common Bio-medical waste treatment and disposal facility.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE No. | TITLE OF ANNEXURE |
| Annexure-I | Hazardous waste receipt Form-3 |
| Annexure-II | Hazardous waste manifest Form-9 |
| Annexure-III | Hazardous waste disposal Record. |
| Annexure-IV | Transport emergency (TREM) card |
ENCLOSURES: SOP Training Record.
- DISTRIBUTION:
| Controlled Copy No. 01 | : | Safety Health & Environment |
| Master Copy | : | Quality Assurance Department |
- ABBREVIATIONS:
| EHS | : | Environment Health and Safety. |
| SOP TREMSHE: | : : : | Standard Operating Procedure Transport Emergency Card Safety, Healthy & Environment |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To be written manual |
ANNEXURE-I
HAZARDOUS WASTE RECEIPT FORM-3
ANNEXURE-II
HAZARDOUS WASTE MANIFEST FORM-9
ANNEXURE-III
HAZARDOUS WASTE DISPOSAL RECORD
ANNEXURE-IV
TRANSPORT EMERGENCY (TREM) CARD
Frequently Asked Question?
- Q: What is hazardous waste?
- A: Waste harmful to health or the environment, requiring special disposal to become harmless or less dangerous.
- Q: What are some examples of hazardous waste generated?
- A: Daily sweepings, QC sample leftovers, off-spec raw materials/products, expired materials, rejected products, stability samples, expired QC samples, microbiology culture waste, and any other identified hazardous waste.
- Q: What form is used to document hazardous waste collection?
- A: Form-3 (Hazardous Waste Receipt) following Annexure-I.
- Q: How are some hazardous wastes deactivated?
- A: By Using 4% Sodium Hypochlorite solution for 48 hours as per Annexure-II.
- Q: What PPE is required for handling hazardous waste?
- A: Personnel protective equipment must be used during collection and transfer.
- Q: How are hazardous wastes prepared for disposal?
- A: Milled/defaced if required, accumulated until a full truckload/container, and then disposed of.
- Q: Where are hazardous wastes disposed of?
- A: To cement units/TSDF with Form-9 (Hazardous Waste Manifest) and Form-10 (TREM Card), following Annexure-II.
- Q: How is hazardous waste disposal recorded?
- A: Quantities received and disposed of are recorded in the “Hazardous Waste Disposal Record” (Annexure-III), and manifests are filed with acknowledged copies.
- Q: How is microbiology culture waste disposed of?
- A: To a common biomedical waste treatment and disposal facility.
