Computer System Validation (CSV)
Computer System Validation (CSV) in the pharmaceutical industry is a critical process to ensure the reliability and accuracy of computerized systems used in drug development and manufacturing. It involves a series of activities to confirm that these systems meet predefined specifications and fulfill their intended purpose. CSV helps to maintain data integrity, protect patient safety, and comply with regulatory requirements.
Installation Qualification (IQ)
In Computer System Validation (CSV) in the pharmaceutical industry, Installation Qualification (IQ) is the initial phase of verifying that a computer system is properly installed and configured. IQ focuses on ensuring that the hardware and software components are received as specified by the manufacturer, installed correctly in the designated environment, and that all necessary utilities and environmental conditions are met. This involves checking for the presence of all components, verifying serial numbers, and ensuring proper connections and cabling. IQ establishes a baseline for subsequent validation activities, confirming that the system is ready for further testing and operational use.
Operational Qualification (OQ)
Operational Qualification (OQ) in Computer System Validation (CSV) in the pharmaceutical industry is the second phase of the validation process. OQ verifies that the computer system functions as intended according to its specifications and user requirements. This involves testing the system’s functionality, accuracy, and reliability. OQ ensures that all system parameters, alarms, and safety features operate correctly. This phase confirms that the system is capable of performing its intended tasks consistently and reliably, providing confidence that it will deliver accurate and trustworthy results.

Key Parameter of Below Page:
Table of Content
Find below pages for complete protocol:

Key Parameter of Below Page:
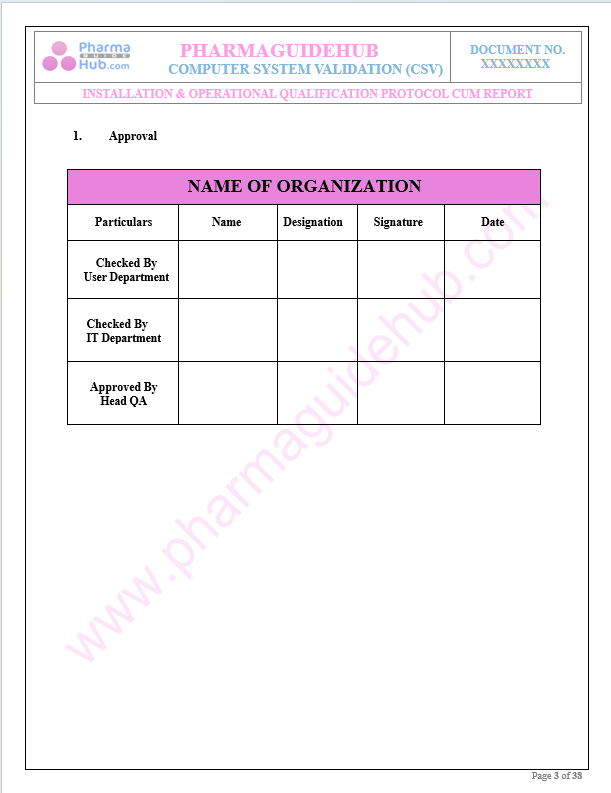
Approval
Find below pages for complete protocol:
Key Parameter of Below Page:
Purpose
Scope
Description
Find below pages for complete protocol:

Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/
Key Parameter of Below Page:
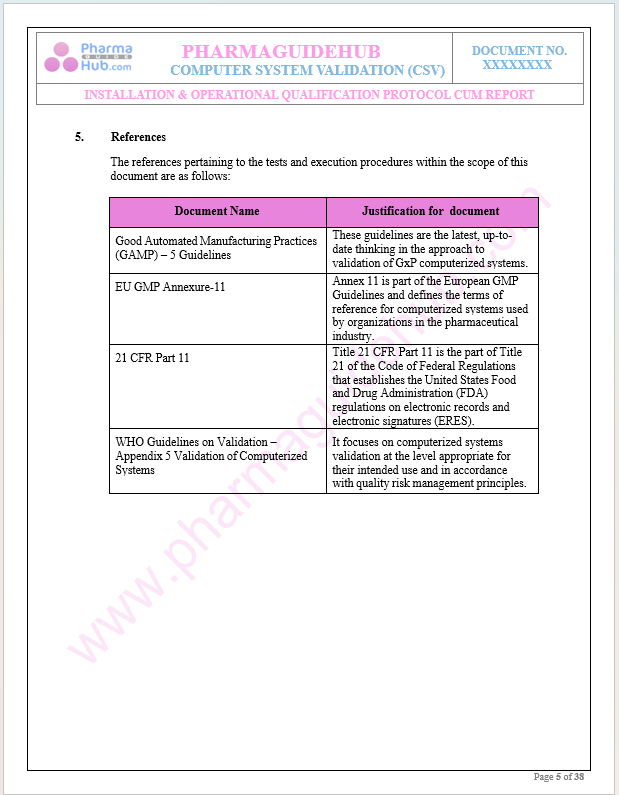
References
Find below pages for complete protocol:
Key Parameter of Below Page:
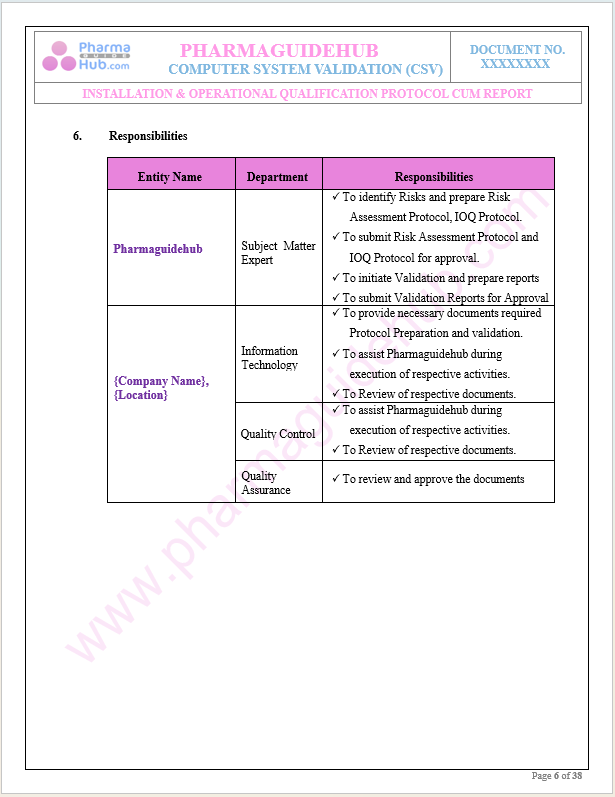
Responsibilities
Find below pages for complete protocol:
Key Parameter of Below Page:
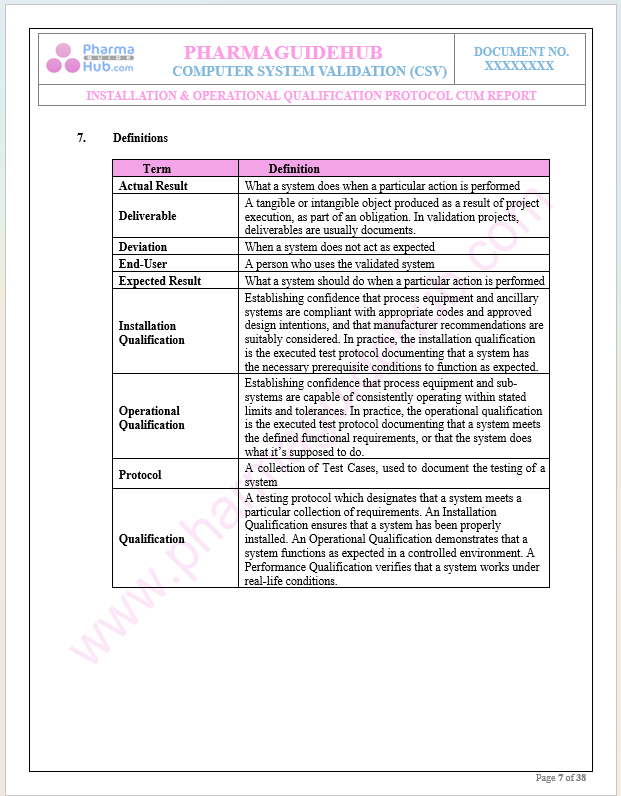
Definitions
Find below pages for complete protocol:
Key Parameter of Below Page:
Definitions
Find below pages for complete protocol:

Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/
Key Parameter of Below Page:
Execution Instructions
Find below pages for complete protocol:
For the Execution of Computer System Validation Please write us at pharmaguidehub@yahoo.com

Key Parameter of Below Page:
Installation Qualifications
Find below pages for complete protocol:

Key Parameter of Below Page:
Installation Qualifications
Find below pages for complete protocol:
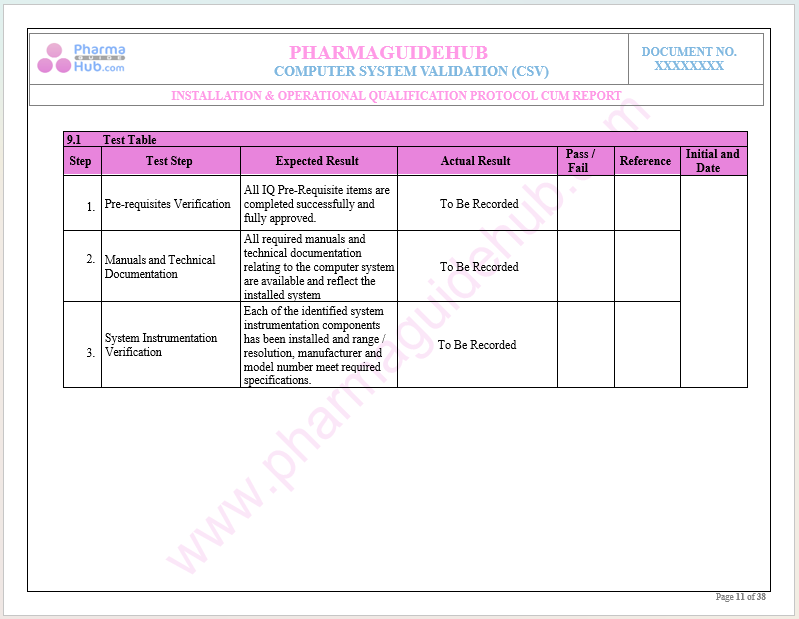
Key Parameter of Below Page:
Installation Qualifications
Find below pages for complete protocol:
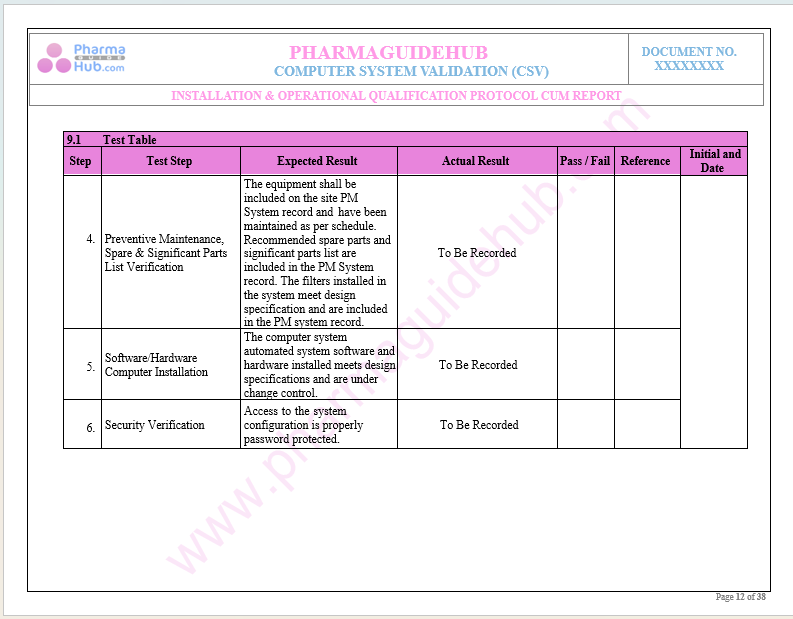
For the Execution of Computer System Validation Please write us at pharmaguidehub@yahoo.com
Key Parameter of Below Page:
Installation Qualifications
Find below pages for complete protocol:
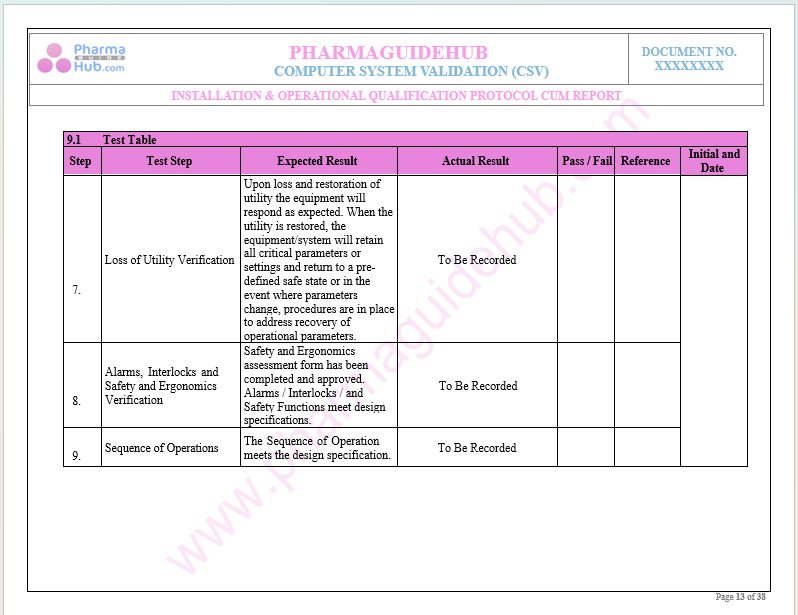
Key Parameter of Below Page:
Record Verification During Installation Qualifications
Find below pages for complete protocol:

Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/
Key Parameter of Below Page:
Record Verification During Installation Qualifications
Find below pages for complete protocol:
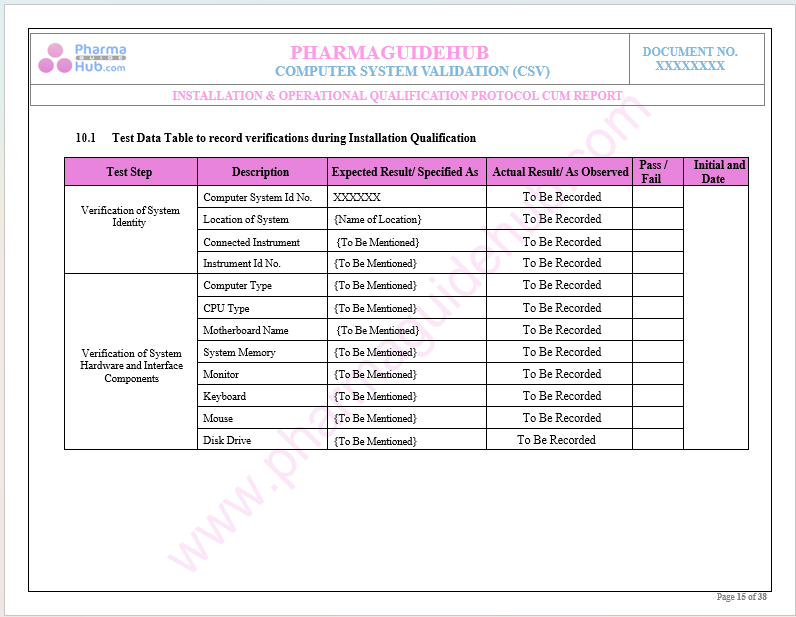
Key Parameter of Below Page:
Record Verification During Installation Qualifications
Find below pages for complete protocol:
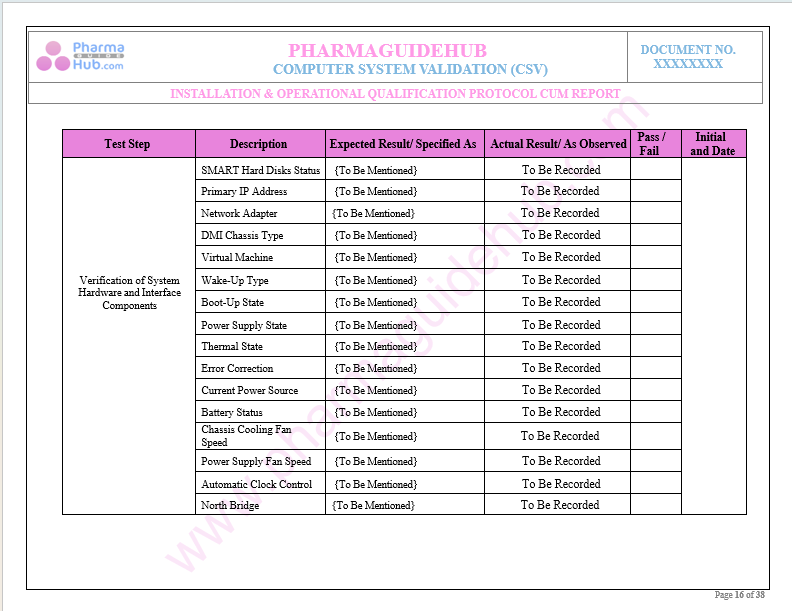
Key Parameter of Below Page:
Record Verification During Installation Qualifications
Find below pages for complete protocol:
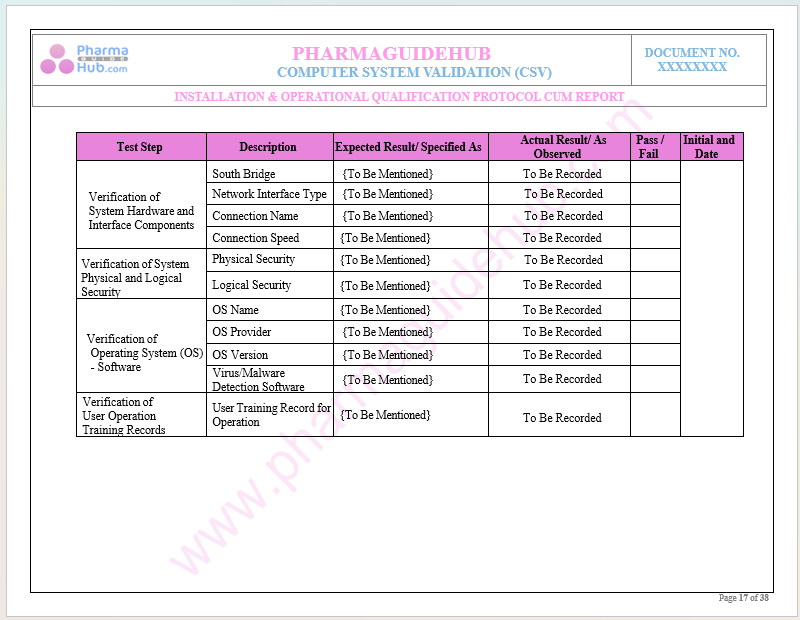
Key Parameter of Below Page:
Record Verification During Installation Qualifications
Find below pages for complete protocol:
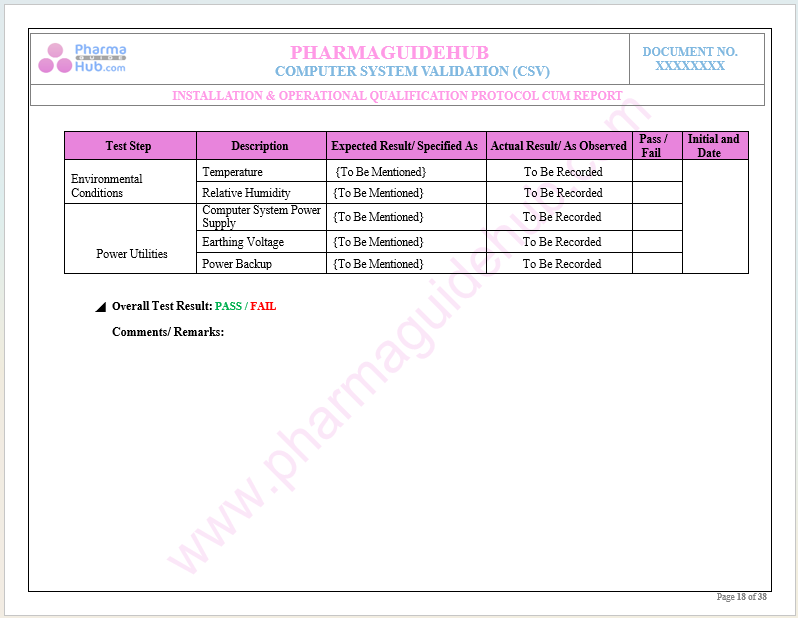
Key Parameter of Below Page:
Operational Qualification
Find below pages for complete protocol:

For the Execution of Computer System Validation Please write us at pharmaguidehub@yahoo.com
Key Parameter of Below Page:
Operational Qualification
Find below pages for complete protocol:
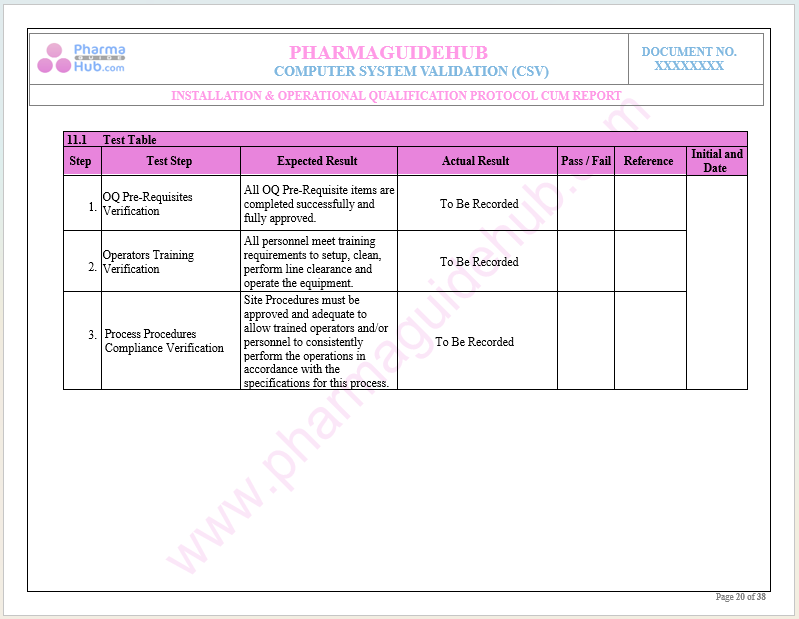
Key Parameter of Below Page:
Operational Qualification
Find below pages for complete protocol:
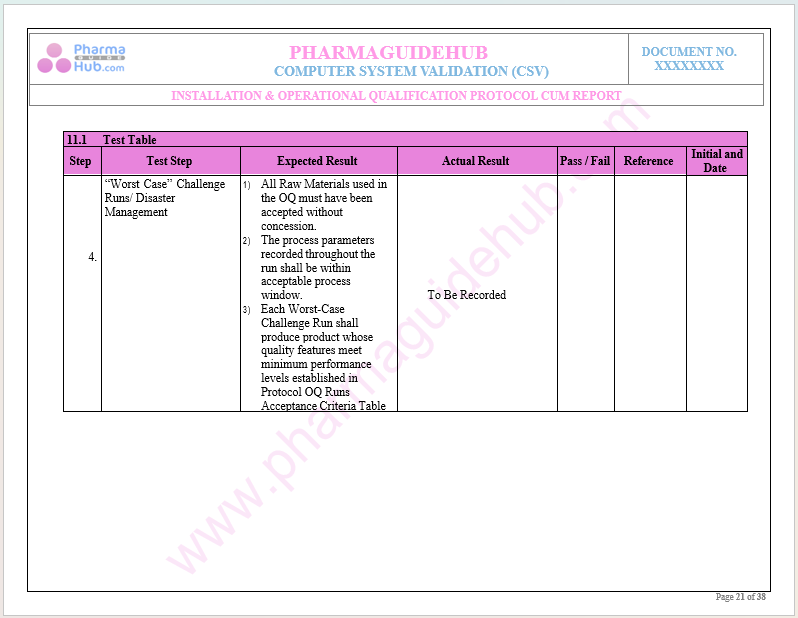
Key Parameter of Below Page:
Record Verification During Operational Qualification
Find below pages for complete protocol:

Key Parameter of Below Page:
Test Data Verification During Operational Qualification
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/
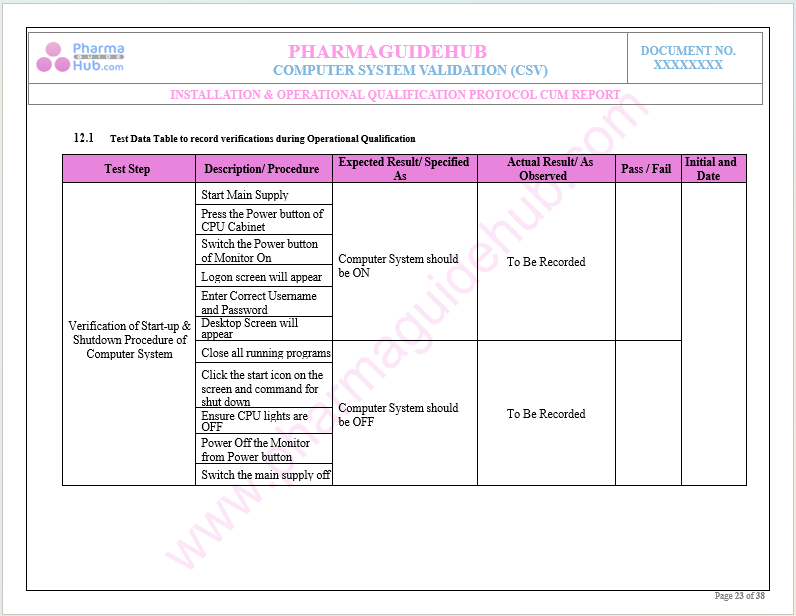
Key Parameter of Below Page:
Test Data Verification During Operational Qualification
Find below pages for complete protocol:
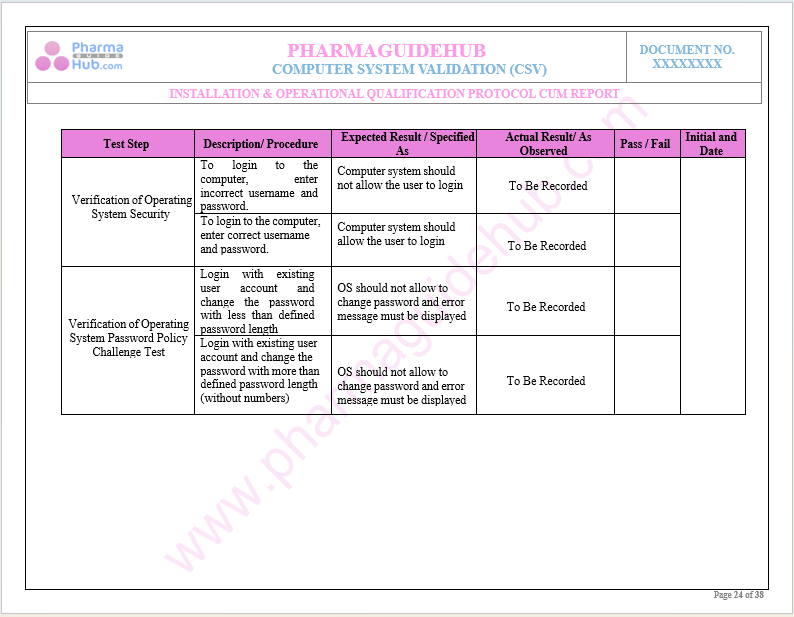
Key Parameter of Below Page:
Test Data Verification During Operational Qualification
Find below pages for complete protocol:
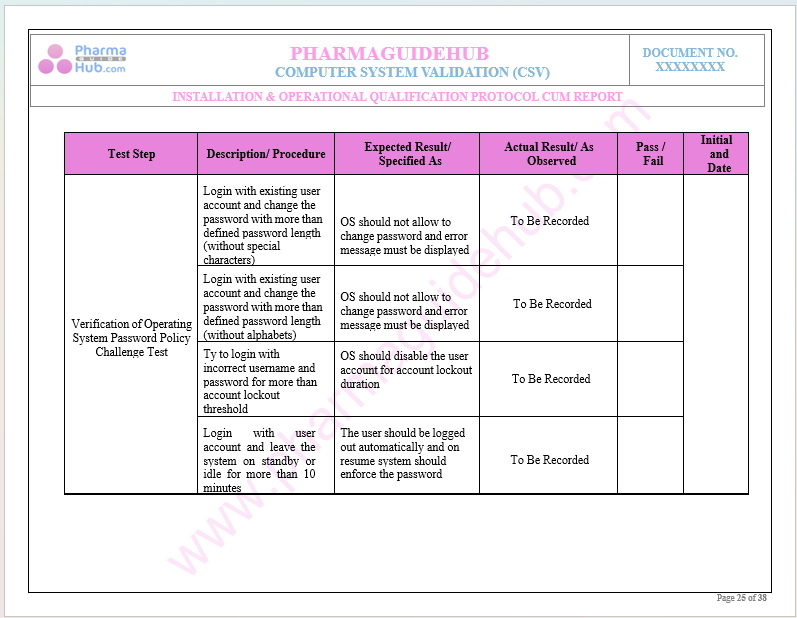
Key Parameter of Below Page:
Test Data Verification During Operational Qualification
Find below pages for complete protocol:
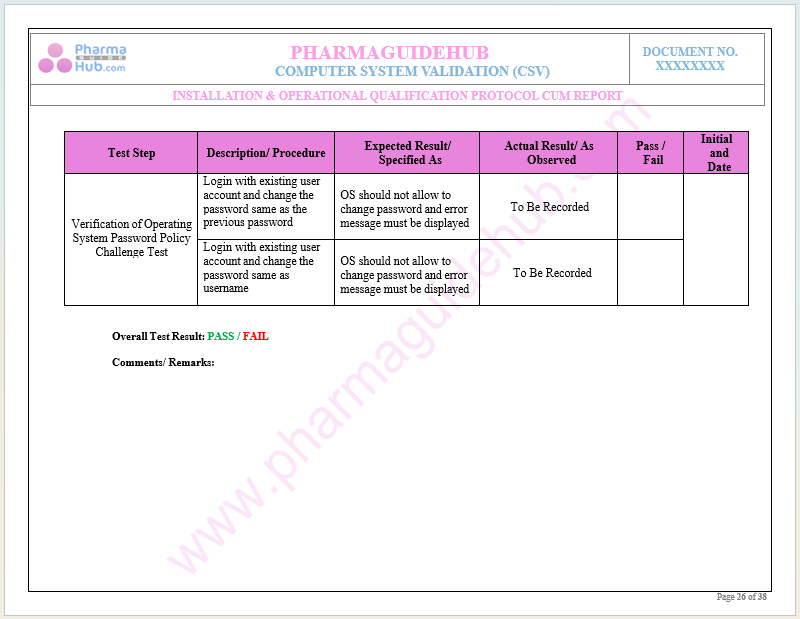
Key Parameter of Below Page:
Verification of SOP and Disaster Recovery Plan Policy
Find below pages for complete protocol:

Key Parameter of Below Page:
Verification of SOP
Find below pages for complete protocol:
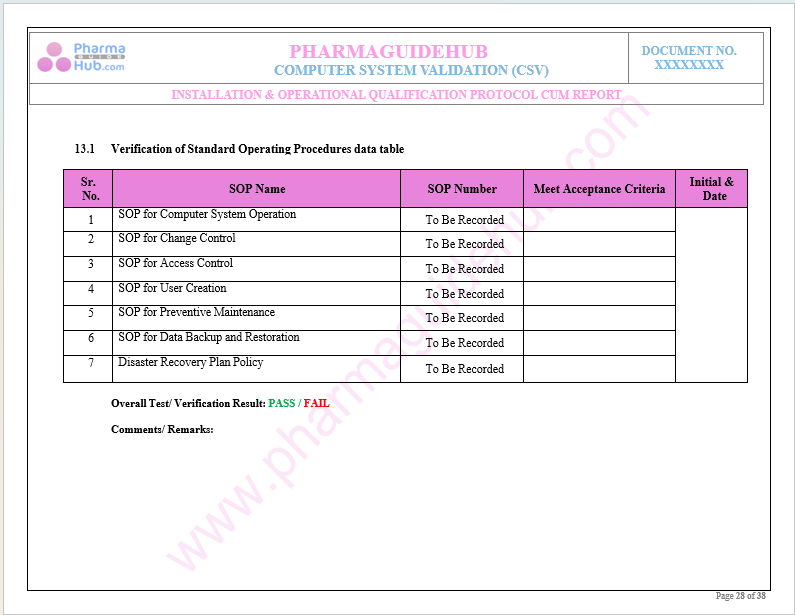
Key Parameter of Below Page:
Computer System Validation Test Status
Find below pages for complete protocol:
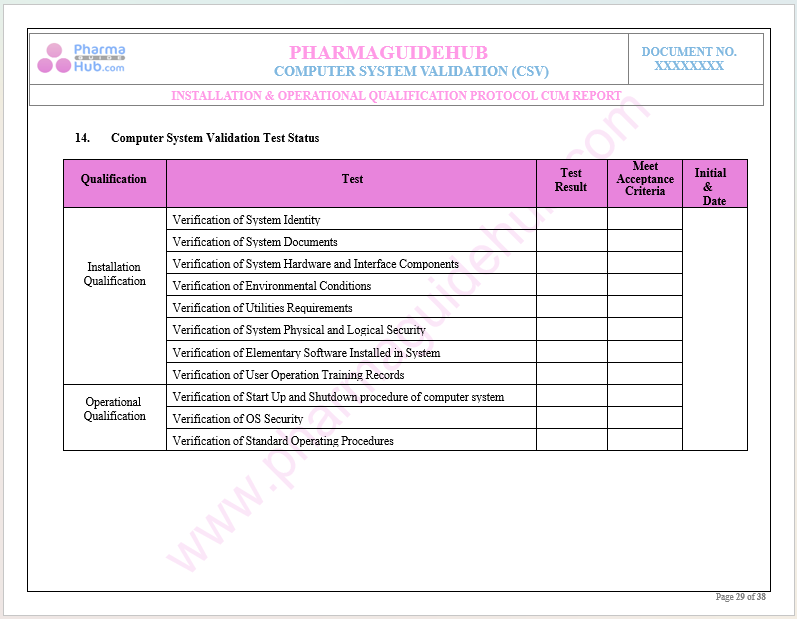
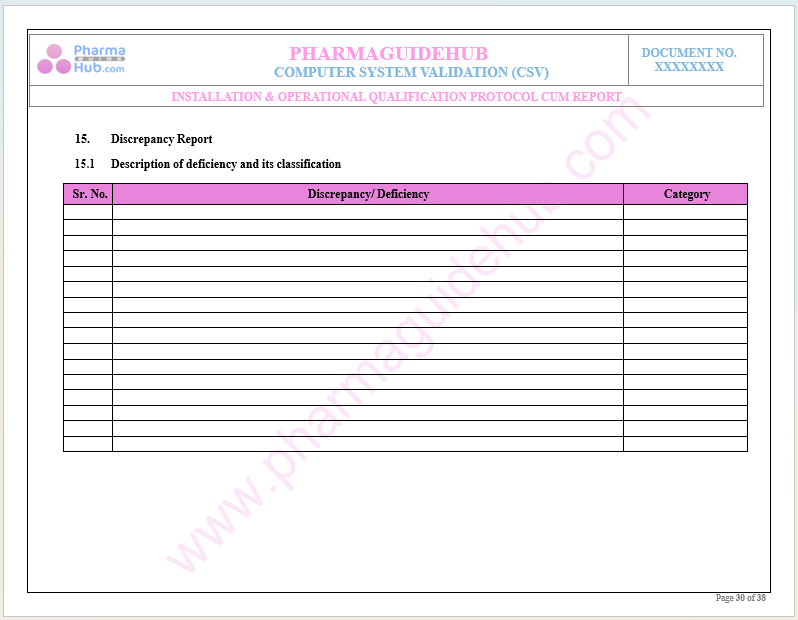
Key Parameter of Below Page:
Discrepancy Report
Find below pages for complete protocol:
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/
Key Parameter of Below Page:
Recommended Corrective Action Plan and Responsible Person
Find below pages for complete protocol:
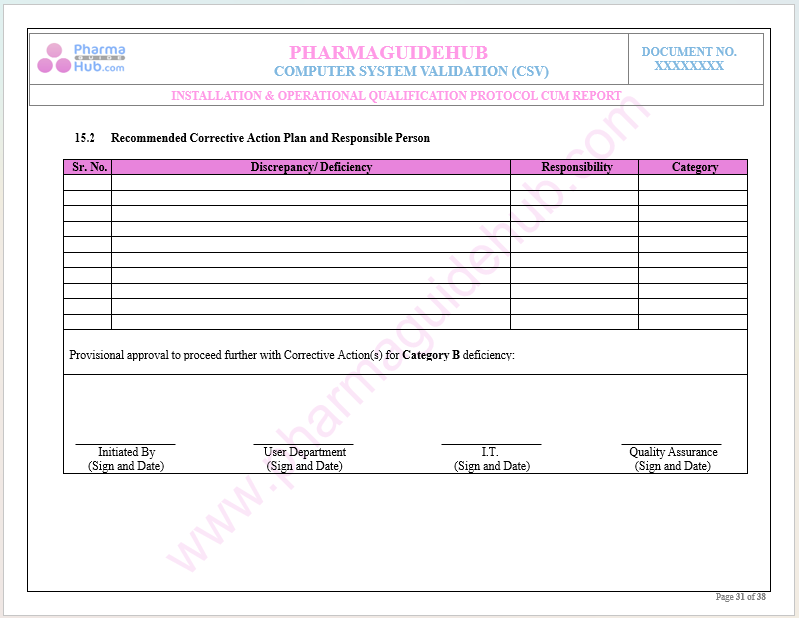
Key Parameter of Below Page:
Corrective Action Taken
Find below pages for complete protocol:

Key Parameter of Below Page:
Recommended Corrective Action Taken within Stipulated Period
Find below pages for complete protocol:
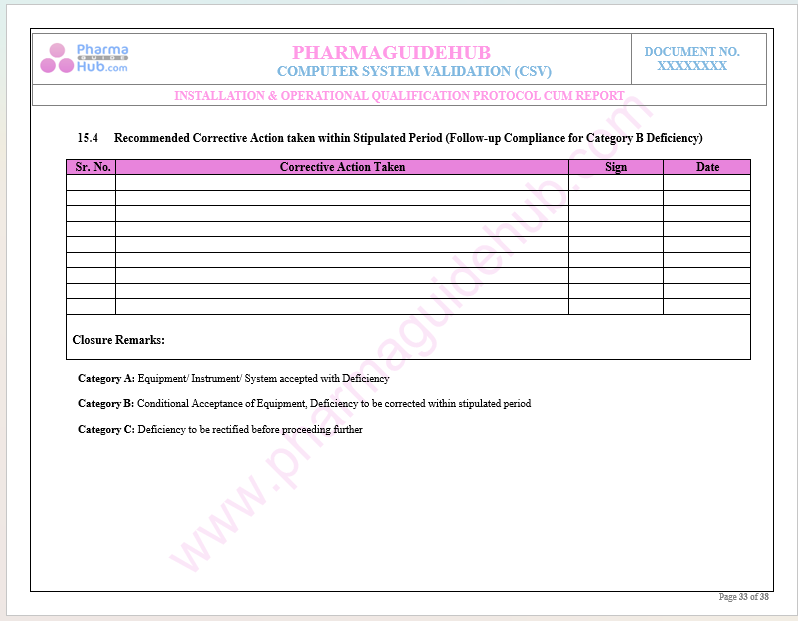
For the Execution of Computer System Validation Please write us at pharmaguidehub@yahoo.com
Key Parameter of Below Page:
Acceptance Criteria
Summary
Conclusion
Find below pages for complete protocol:

Key Parameter of Below Page:
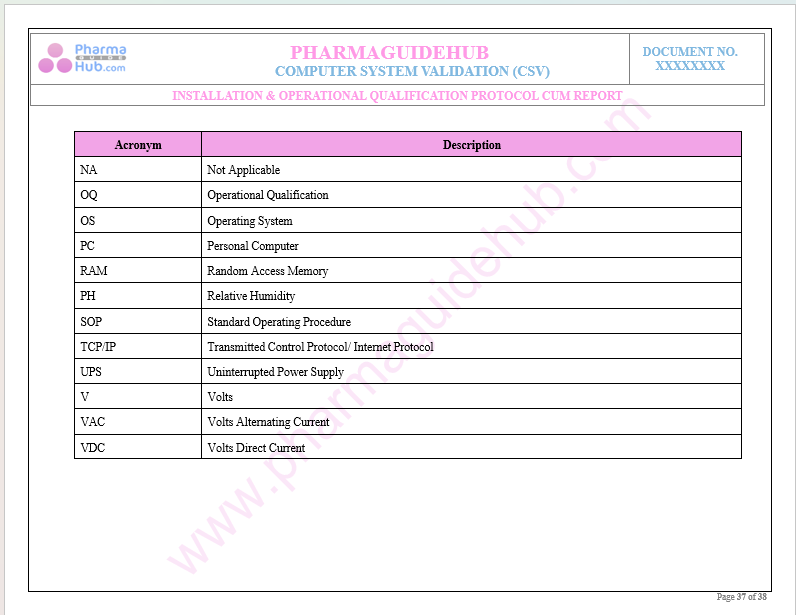
Abbreviations
Find below pages for complete protocol:
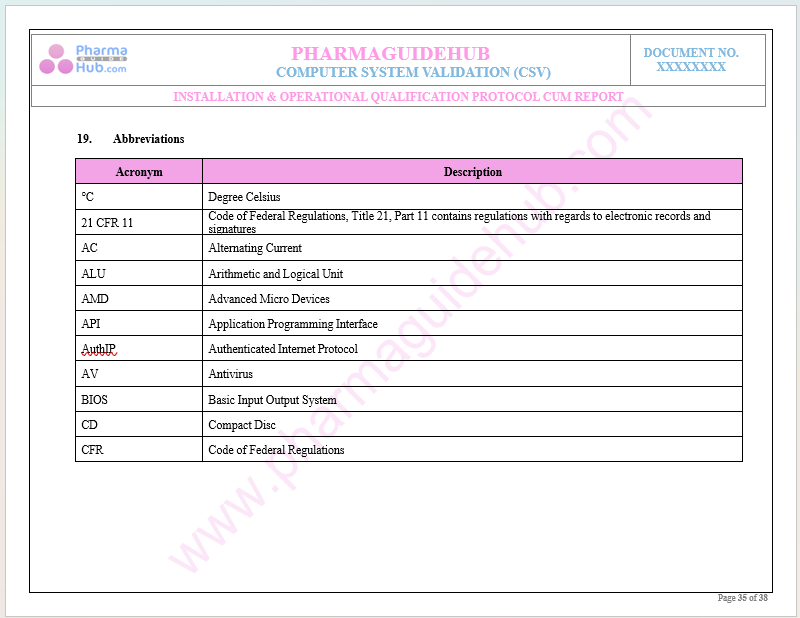
Key Parameter of Below Page:
Abbreviations
Find below pages for complete protocol:
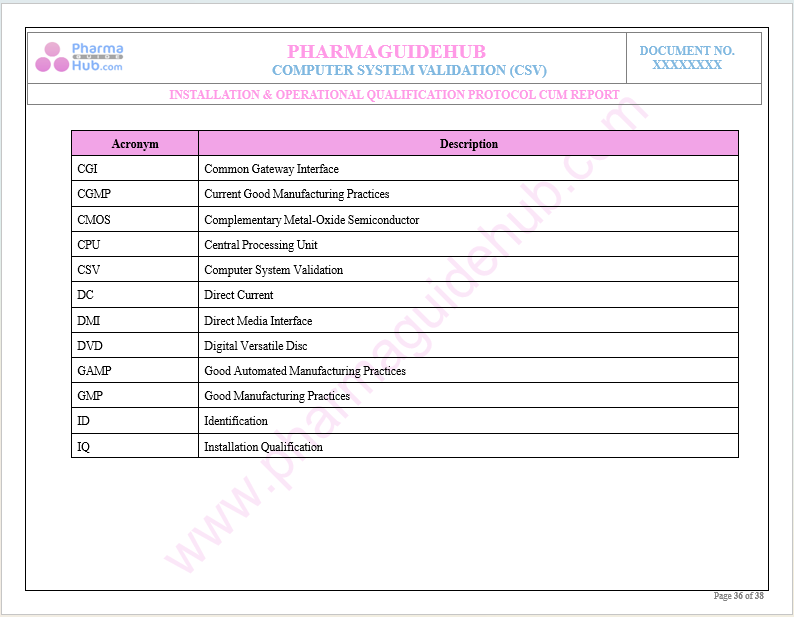
Key Parameter of Below Page:
Abbreviations
Find below pages for complete protocol:
Key Parameter of Below Page:
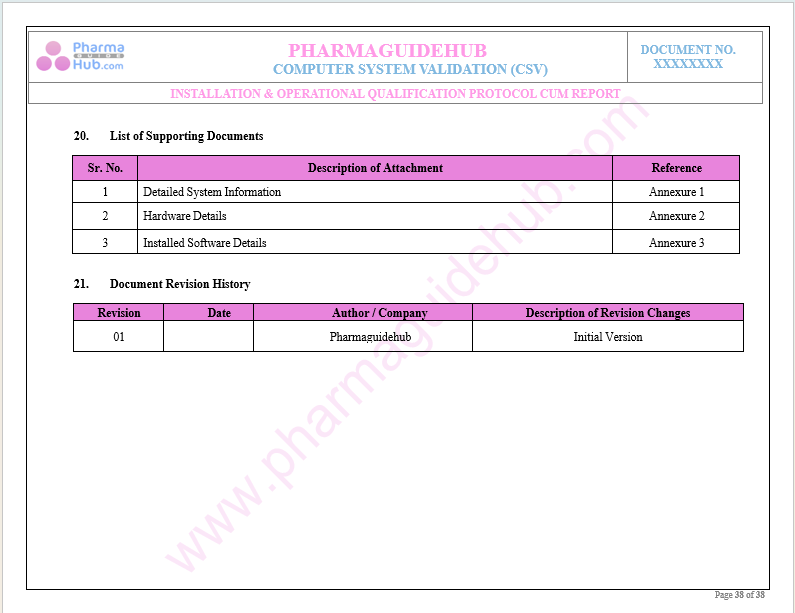
List of Supporting Documents
Document Revision History
Find below pages for complete protocol:
For the Execution of Computer System Validation Please write us at pharmaguidehub@yahoo.com
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/computer-system-validation-csv-installation-operational-qualification-protocol-cum-report/





