OBJECTIVE:
The objective of this document is to define the requirements for the Cross Contamination Control Program.
SCOPE:
This Policy is applicable for cross contamination control at {Company Name} {Location}.
DEFINITIONS:
The definitions listed here apply in the context of this Policy Document as well as for other documents used for Cross Contamination Control and may or may not be applicable in all other usage.
- Acceptance Criteria – The criteria i.e. a system/ product/ process must meet to successfully complete a test phase or to achieve delivery requirements.
- Acceptable Daily Exposure (ADE) – A dose that is unlikely to cause an adverse effect if an individual is exposed, by any route, at or below this dose every day for a lifetime.
- Action Limit – A limit that when reached requires an action to bring the process back under control.
- Alert Limit – A limit that when reached alerts the staff that the process may be trending out of control.
- Dedicated Manufacturing Facility – A facility, which can be a separate building or a separate area within a building, which is dedicated to the manufacture of a class of compounds (hormone, etc.) or a specific API or drug product.
- Health-Based Limit – A limit based on the health risks to patients as determined by a toxicological and pharmacological assessment. Also known as, limit determined from the acceptable daily exposure (ADE) or permitted daily exposure (PDE).
- Maximum Allowable Carry Over (MACO) – Maximum amount of the residue of the previous product which is considered safe if passed into the next product batch. This is also termed as the Maximum Allowable Residue (MAR).
- Margin of Safety – The distance between the highest data point and acceptance criteria Permitted.
- Daily Exposure (PDE) – A substance-specific dose that is unlikely to cause an adverse effect if an individual is exposed at or below this dose every day for a lifetime.
- Process Control Limit – A limit established based on statistical analysis of the data.
- Product – A finished dosage form (tablet, capsule, vial, etc.) that contains one or more active pharmaceutical ingredients and possibly inactive ingredients. Products can either be made in commercial batches, or development batches for scale up or exhibition (submission). Product
- Dedicated Modules – A suite or portion of a facility that is segregated and dedicated to a product or group of products.
- Quality Risk Assessment – A systematic process for the assessment (identification, analysis and evaluation) of risks to the quality of the drug product across the product life cycle. Quality
- Risk Management – A proactive means to identify and control potential quality issues during research, development, manufacturing, and distribution of a product or service. The systematic application of management policies, procedures and practices to the tasks of analyzing, evaluating and controlling risk. Specifically, a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle.
- Quality Risk Management Exercise – A Quality Risk Management project focused on a specific event or events.
- Routine Monitoring – The process of confirming the systems (risk controls) are working as specified during routine operations.
- Segregated Facility – A segregated or self-contained facility is either a separate building or an area within a building that is completely physically separated (floor to floor walls) with one or more rooms with its own air-locked personnel/material access, HVAC systems, processes, equipment and cleaning procedures.
- Therapeutic Dose – An amount of drug that will produce a pharmacological response.
- Verification – The periodic process of confirming the risk control effectiveness through data gathering and analysis as specified in a protocol study. This process shall be documented in a report.
- Visually Clean Criterion – Verification of absence of any visible residues such as drug product, dusts, liquids, oils or cleaners after cleaning under normal workspace lighting conditions when inspected with the eyes.
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/cross-contamination-control-policy/
DOCUMENTATION REQUIREMENTS
- Procedure for Quality Risk Management
- Procedure for cleaning validation program.
- Site procedure for new product assessment
- Site procedures for mix-up control (E.g. labelling control, line clearance etc.)
- Site procedure for cleaning of equipments, area, filter and gowns.
- Site procedure for man, material and equipment movement
- Site procedure for gowning and de-gowning
- Site procedure for filter monitoring/replacement
- Site procedure for monitoring pressure differential
POLICY
General Considerations:
In order to manufacture products in shared facilities, health-based limits shall be established, analytical methods with sensitivity to detect relevant residue levels and operational/technical controls to manage the risk of cross contamination shall be in place. If any of these are not in place, dedicated and segregated manufacturing facilities shall be required.
If a facility is dedicated to a class of compounds such as hormones, potent materials, etc., the requirements for the use of shared facilities shall be met.
The manufacture of finished drug products shall be complimented by risk-based control measures which are scientifically derived and appropriately suited to manage the risk of cross-contamination to a pre-defined level of acceptability.
Health-based limits (ADE/PDE) shall be established and utilized to assist in determining the appropriateness of spatial, structural, operational, and procedural controls which are established.
All control strategies shall include a periodic re-validation assessment and possess multiple product detection areas – with established limit associated with each.
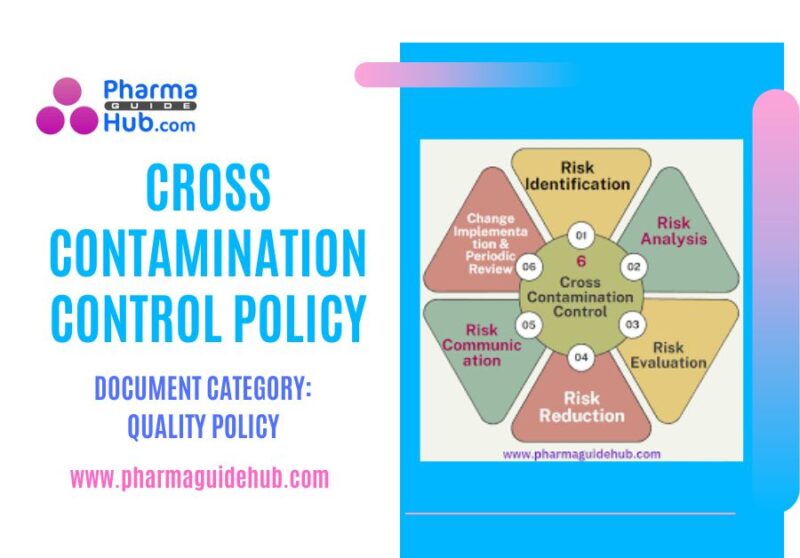
Cross Contamination Control:
A formal assessment of the control measures required to accompany new Active Pharmaceutical Ingredient (API) and finished drug product shall be conducted prior to introduction of the material and product into the product matrix of an existing manufacturing operation.
All existing facilities should conduct an assessment of the current operation.
A formal assessment of the controls required to accompany existing compounds shall be integrated into the design of all new manufacturing operations.
Assessments shall address the potential for mix-up, retention/carryover of residues from one product into another after cleaning of shared equipment, mechanical transfer of residues from non-product contact surfaces into or onto another product, and airborne transfer of residues from aerosols that sediment into or onto another product.
The tools which are utilized to identify risks for cross-contamination shall be consistent with established methodologies for such determinations.
Prior to the initiation of manufacturing activities, a health-based limit (ADE/PDE) shall be generated and subsequently utilized to assist in the development of a scientifically defensible control strategy.
The adequacy of controls which are developed shall be formally embedded and assessed in initial Process Validation activities and remain an integral component of all subsequent revalidation efforts.
Where applicable, the dynamic verification of the continued adequacy of controls must be established as a part of post FMEA data gathering. Multiple areas associated with the pre-established presence of a given material of interest in a specific area shall be identified, monitored and assigned Alert and Action limits.
Documentation: Assessment for Cross Contamination Control shall be documented in QRM Evaluation Form.
Training Aspects: Personnel shall be adequately trained in Quality Risk Management and on how cross contamination occurs, how to control cross contamination and how cross contamination affects product quality.
Cross Contamination Control Failure Considerations:
Notification to management or escalation methodology shall be formally established and utilized in the event of a failure in pre-defined controls. A maximum timeframe associated with such notification(s) to occur shall be formally integrated into the escalation process.
Any failure in established controls shall trigger an assessment through process non-conformance which incorporates both a review of other batches of the same product and extends to batches of other products potentially impacted by the failure.
A failure investigation shall be conducted, root cause or probable cause shall be determined, and an adequate control measure shall be put in place.
Review and Revision:
Whenever following changes are necessitated, an Impact Assessment for evaluating the requirement of re-validations and/or re-assessment of the cross contamination control procedures shall be done:
- Addition of new product
- Change in facility or equipment or process
- Change in batch sizes and/or frequency of manufacture
- Additional data collection
If none of the above occurs, the risk management exercise shall be reviewed for adequacy as per defined frequency.
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New policy introduced | Not Applicable | To Be Written Manual |
Click the link to download word file copy of this document:
https://pharmaguidehub.com/product/cross-contamination-control-policy/