- PROCEDURE:
- Login the Client computer with the assigned user ID and password.
- Locate the Data path where the instrument generated data being saved by the software.
- Create a folder month wise as “(ex…January_2025)” in the Local D or E drive .
- Create the subfolders as “Instrument ID” in the monthly folder, copy the relevant data from the original location of the software.
- Verify the Copied data to ensure all the data has been captured from the original location.
- Check the size of the monthly folder and accordingly choose the backup media CD/DVD.
- Copy the backup Data from all the local PC’s to the file server share folder “QC Equipment Data” as a centralized backup location.
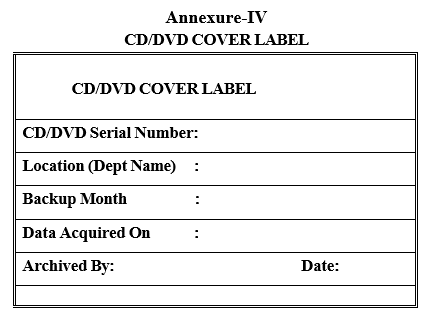
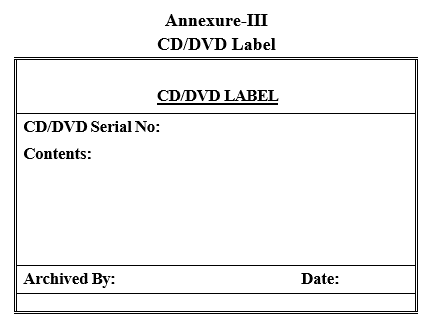
- Archive the data from the share folder to CD/DVD, Label the CD/DVD as per Format-III and CD/DVD cover as per Format-IV.
- Backup of Audit trails/History shall also be archived along the data for the applicable Software’s.Backup and archival shall be done last working day of every month within +/-2 Days.
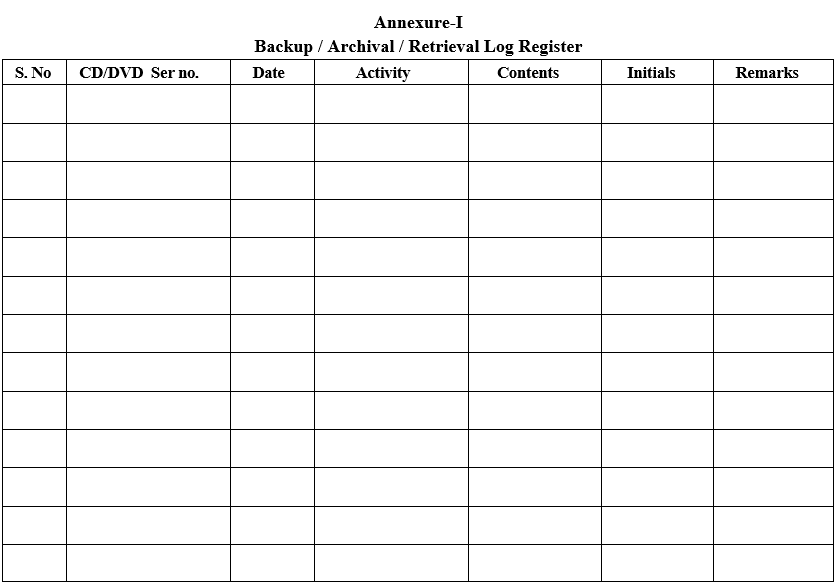
- Enter the backup/Archival/Retrieval details in Format -I.CD/DVD Serial No shall be Numbered as Ex..(DVD (Backup Media)/ARC (Archival)/001(First DVD)/11(Year)).
- After Archival verify the data in the CD/DVD whether all the data has been Captured, then handover to the documentation cell.
- One more copy of the Backup data shall be maintained in the File server share folder “QC Equipment Data”.
- The last archived data shall be verified once every three months by opening some relevant files randomly from the backup media, enter the details in Format-I.
- The above three months data shall be removed from the local PC after verification of the Archived Data.
- The Backup data shall be restored into the client upon request as per Format -II.
- If any problem is occurred during backup/Archival/Restoration process, the matter shall be intimated to the suppler for necessary support and action.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Backup / Archival / Retrieval Log Register |
| Annexure-II | Client Data Requisition Request |
| Annexure-III | CD/DVD Label |
| Annexure-IV | CD/DVD Cover Label |
- ABBREVIATIONS:
| No. | : | Number |
| CD | : | Compact Disc |
| DVD | : | Digital versatile Disc |
| CD-R | : | Compact Disc Readable |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Annexure-I
Backup / Archival / Retrieval Log Register
Annexure-II
Client Data Requisition Request

Annexure-III
CD/DVD Label
Annexure-IV
CD/DVD COVER LABEL