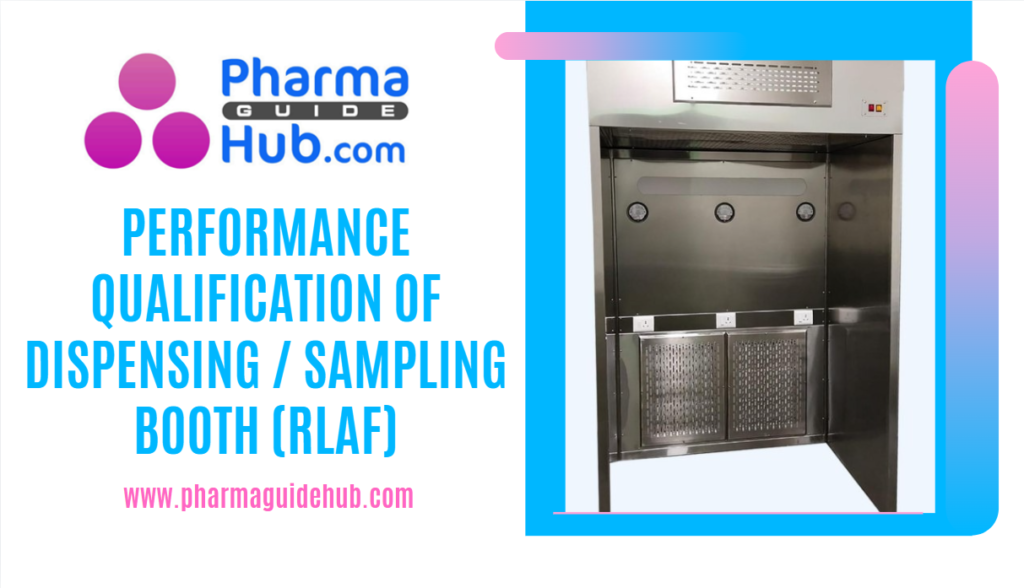
Reverse Laminar Airflow (RLAF), also known as a Sampling and Dispensing Booth, is a specialized piece of equipment widely used in the pharmaceutical industry to provide a clean, controlled environment for handling powders and other particulate materials.
Applications of RLAF in Pharmaceutical Manufacturing
- Powder Handling: RLAF booths are commonly used for tasks such as weighing, dispensing, and transferring powders.
- Sampling: They can also be used for sampling pharmaceutical products to ensure quality and consistency.
- Aseptic Processing: RLAF can be used in areas where aseptic conditions are required, such as the filling of sterile products.
Key aspects of Reavers laminar airflow qualification:
Evaluate the system’s performance during routine operations and under simulated worst-case scenarios.
Installation Qualification (IQ):
Verify the correct installation of the Reaver unit according to manufacturer’s specifications and site requirements.
Check the integrity of electrical connections, air supply, and exhaust systems.
Ensure that the unit is properly grounded.
Operational Qualification (OQ):
Verify that the Reaver system operates as intended under specified conditions.
Test the airflow rate, velocity, and uniformity within the working zone.
Evaluate the system’s ability to maintain temperature and humidity levels.
Check the performance of HEPA filters and other filtration components.
Performance Qualification (PQ):
Assess the Reaver’s ability to maintain a controlled environment under actual operating conditions.
Monitor particle counts within the working zone to verify the system’s effectiveness in preventing contamination.
Conduct microbial monitoring to assess the system’s ability to control microbial growth.