- OBJECTIVE:
To establish the process for classification of Events based on Risk Assessment.
- SCOPE:
This SOP is applicable to the events managed/reported through the Process Non Conformance (PNC) [triggered from Audit observations, Calibration failures, Documentation Errors, Equipment/Utility failures, Labeling errors, Validations, Annual Product Review, Analytical failures, Trend Analysis / OOT, Environmental/Microbiological failures, etc.]. Material Non Conformance (MNC), Out of Specification (OOS) at {Company Name} {Location}.
- RESPONSIBILITY:
- The personnel identifying/initiating the Event shall be responsible to describe and classify the Event.
- Department Head/QA Reviewer shall be responsible to ensure the correctness of description and the classification of Event and Modify the description of Event and/or re-classify the Event, if required.
- ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
- PROCEDURE: An Event is an occurrence or incidence which does not comply with the written specification or procedure.
- Event Description:
- Event shall be clearly understood and described in respective relevant documents (as applicable) prior to classification of the Event.
- Event description shall clearly indicate what actually happened and what should have happened.
- Event Description:
- Event Classification: The Event shall be classified based on the severity and the risk associated with the Event, as follows:
- Critical: An Event that has a confirmed impact on the regulatory requirements, quality, potency, safety, effectiveness, or fitness for use of the product. For example, the failure of a finished product lot or intermediate to meet specification (including in-process specifications) is a critical Event.
Click the link to download word file copy of this document: https://pharmaguidehub.com/product/event-classification/
A critical event shall be considered as Quality Impacting Event for non-conformance classification.

- Major: An Event that has a moderate to high likelihood of impacting the regulatory requirements, quality, potency, safety, effectiveness, or fitness for use of the product.
- Minor: An event that is unlikely to impact the regulatory requirements, quality, potency, safety, effectiveness, or fitness for use of the product.
A Major/Minor event shall be considered as Non-Quality Impacting Event for non-conformance classification.
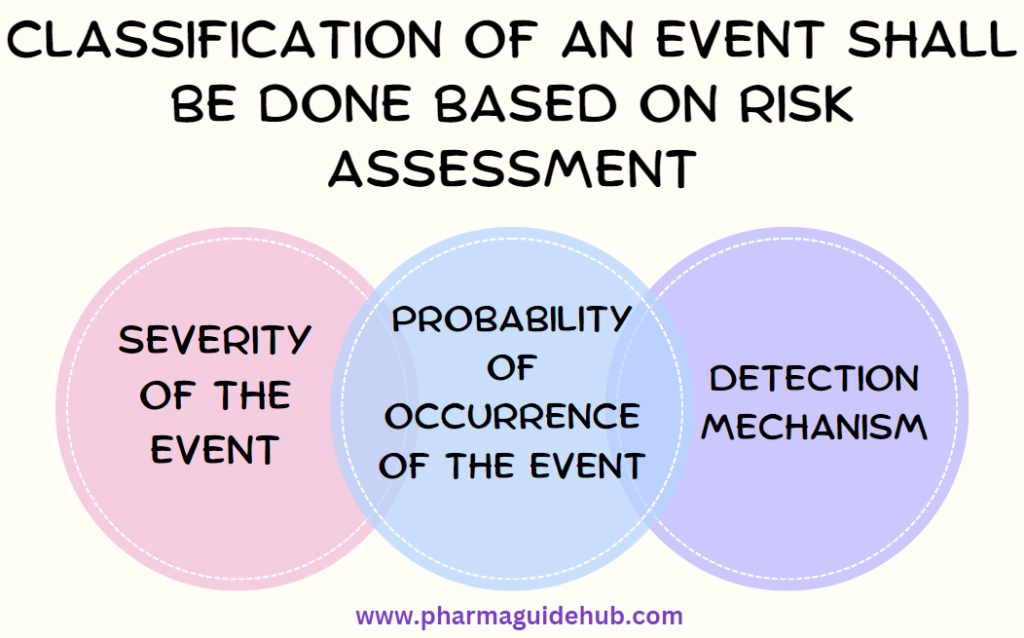
- Classification of an Event as Critical, Major or Minor shall be done based on Risk Assessment considering the following risk factors;
- Severity of the Event
- Probability of Occurrence of the event
- Detection Mechanism
- Severity of the Event:
- Severity of an Event shall be evaluated by assessing the impact of the event on SISPQ of product, process or any other Quality System.
- Ranking of Severity shall be done based on the following
- Severity of the Event:
| Rank | Severity |
| 1 | No effect on output |
| 2 | Minor effect |
| 3 | Moderate effect |
| 4 | Serious effect |
| 5 | Hazardous effect |
Note: F Any Out of Specification result in a GMP laboratory, Critical Utilities Failures, Significant compliance related Events, and/or significant failure of a Quality System shall be considered as serious effect in the severity ranking, regardless of product impact.
- Probability of Occurrence of the Event: Occurrence ranking shall be given by assessing the probability of the occurrence of the event, as follows;
| Rank | Severity |
| 1 | Failure unlikely |
| 2 | Remote Failure |
| 3 | Occasional failure |
| 4 | High |
| 5 | Certain failure |
- Detection Mechanism: Ranking shall be given by assessing the level of detection existing in a system, as follows;
Click the link to download word file copy of this document: https://pharmaguidehub.com/product/event-classification/
| Rank | Severity |
| 1 | Flawless detection |
| 2 | Will detect failure |
| 3 | Might detect failure |
| 4 | Almost certain not to detect failure |
| 5 | No detection mechanism exists |
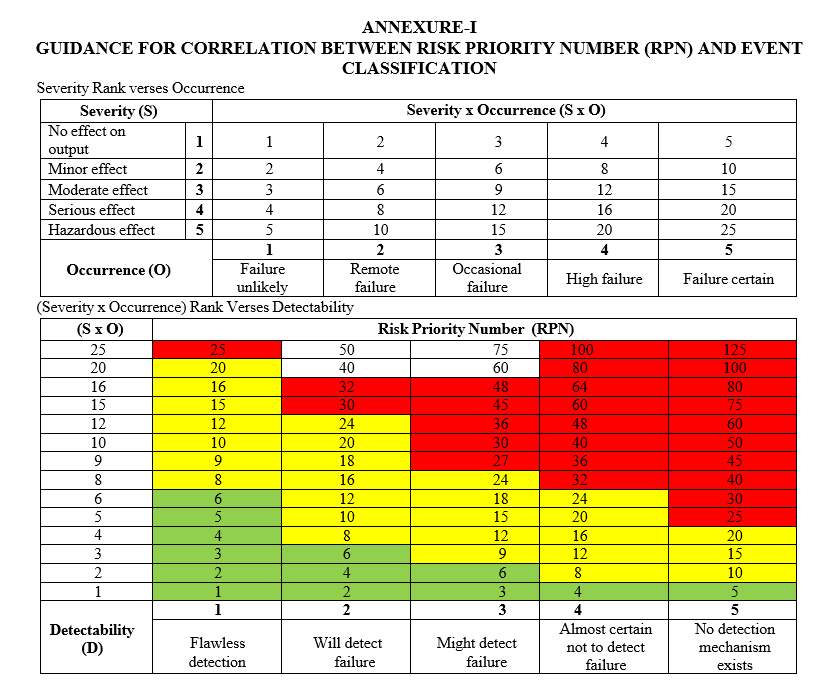
- Risk Priority Number (RPN): The Risk Priority Number shall be obtained by multiplying the Severity, Occurrence and Detectability ranks for an Event.
- The Event shall be classified as Critical, Major and Minor based on the obtained Risk Priority Number (RPN).
- If RPN number of an event is observed to be less than or equal to 6 then the event l shall be considered as Minor; Event with RPN 8 to 24 shall be considered as Major and Event with RPN of greater than or equal to 25 shall be considered as Critical. The same is mentioned in the following table;
| RPN Scale | Category Event classification | |
| ≤6 | The events that are unlikely to have impact on the regulatory requirement and/or SISPQ of the product | Minor |
| 8 – 24 | The events that have a moderate to high likelihood of impact on the regulatory requirement and/or SISPQ of the product | Major |
| ≥25 | The events that have confirmed impact on the regulatory requirement and/or SISPQ of the product | Critical |
Note: Refer Format 1 for the correlation between the Risk Priority Number and Event classification. F In certain Events, the risk of Event can be decided based on the experience of a process and/or an activity and accordingly the Event shall be classified in spite of obtained RPN number. In such cases, the justification shall be provided in the respective Event process
Click the link to download word file copy of this document: https://pharmaguidehub.com/product/event-classification/
- All Events classified as Critical based on this SOP shall be categorized as Quality Impacting in the severity level field for non-conformance classification.
- Events classified as Major or Minor based on this SOP shall be categorized as Non Quality Impacting in severity level field for non-conformance classification.
Wherever the severity is ranked as serious or hazardous and occurrence level is ranked as high failure, failure certain (4 or above), the event shall be categorized as Quality Impacting irrespective of detectability and RPN scale.
- REFERENCES:
Not Applicable
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Guidance for Correlation between Risk Priority Number (RPN) and Event Classification |
- DISTRIBUTION:
- Controlled Copy No. 01 : Head Quality Assurance
- Controlled Copy No. 02 : Head Production
- Controlled Copy No. 03 : Head Warehouse
- Master Copy : Quality Assurance Department
- ABBREVIATIONS:
| RPN | : | Risk Priority Number |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
| CAPA | : | Corrective And Preventive Actions |
| QRM | : | Quality Risk Management |
| SISPQ | : | Safety, Identity, Strength, Purity and Quality |
- REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
Click the link to download word file copy of this document: https://pharmaguidehub.com/product/event-classification/
ANNEXURE-I
GUIDANCE FOR CORRELATION BETWEEN RISK PRIORITY NUMBER (RPN) AND EVENT CLASSIFICATION
Frequently Asked Question?
Q: What is the definition of an “Event” in the pharmaceutical context?
A: An event is defined as an occurrence or incidence that deviates from the written specifications or procedures within the pharmaceutical setting.
Q: How is an event described and documented according to the procedure?
A: The event description is required to be clearly understood and documented in relevant documents before its classification. It should explicitly state what actually happened and what should have happened.
Q: How are events classified, and what criteria are considered for classification?
A: Events are classified based on severity and risk. Critical events have a confirmed impact on regulatory requirements, quality, potency, safety, effectiveness, or fitness for use. Major events have a moderate to high likelihood of impact, while minor events are unlikely to have a significant impact. The classification considers risk factors such as severity, probability of occurrence, and detection mechanisms.
Q: How is the severity of an event evaluated, and what are the severity ranking criteria?
A: The severity of an event is evaluated by assessing its impact on the SISPQ (Safety, Identity, Strength, Purity, Quality) of the product, process, or any other Quality System. Severity ranking is done based on a scale from no effect to hazardous effect, considering factors like Out of Specification results, critical utilities failures, compliance-related events, and significant failures of a Quality System.
Q: What factors are considered for the probability of occurrence of an event?
A: The probability of occurrence is ranked based on the likelihood of the event happening, ranging from failure unlikely to certain failure. This assessment helps in understanding the potential frequency of the event.
Q: How is the Detection Mechanism assessed in the event classification process?
A: The level of detection existing in a system is ranked from flawless detection to no detection mechanism. This factor evaluates the effectiveness of identifying and addressing the event.
Q: What is the Risk Priority Number (RPN), and how is it calculated?
A: The Risk Priority Number is calculated by multiplying the severity, occurrence, and detectability ranks for an event. It serves as a quantitative measure to prioritize events based on their potential impact.
Q: How are events classified based on the obtained Risk Priority Number (RPN)?
A: Events are classified as Critical, Major, or Minor based on the RPN. A table defines the classification: RPN ≤ 6 is Minor, RPN 8-24 is Major, and RPN ≥ 25 is Critical.
Q: How are events categorized as Quality Impacting or Non-Quality Impacting?
A: Critical events, based on this procedure, are categorized as Quality Impacting, while Major or Minor events are classified as Non-Quality Impacting. The severity level is considered in the non-conformance classification process.
Q: Are there exceptions to the RPN-based classification, and how are they justified?
A: In certain cases, the risk of an event can be decided based on process or activity experience, regardless of the RPN number. Justification for such exceptions should be provided in the respective event process documentation.
Click the link to download word file copy of this document: https://pharmaguidehub.com/product/event-classification/