- PROCEDURE:
- Procedure for receiving the Columns:
- Receipt of HPLC & GC Columns along with Certificate of Analysis, supplied by supplier or manufacturer.
- Ensure the physical condition of column at the time of receiving.Columns shall be received along with its literature. 4
- Procedure for Issuance and In-house numbering of the HPLC Column:
- HPLC column shall be issued after receiving the filled request Format as per format-I.
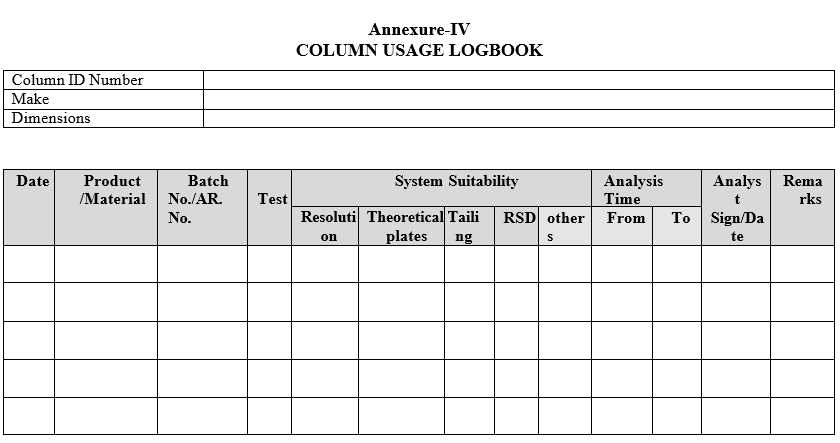
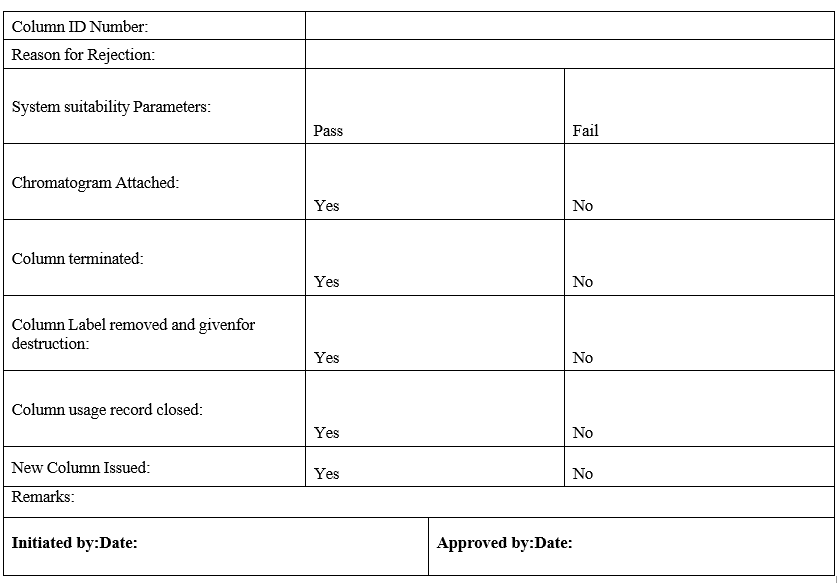
- Perform the “system suitability parameter” as per respective standard test procedure (STP) and record all respective parameters in column usage log per Format-IV & in Column Issuance and Rejection Register as per format-II.
- If HPLC column does not meet the requirement, it shall be rejected and shall be returned to the vendor.
- If HPLC column meets the requirement, then column shall be approved by Section in charge/manager QC.
- The executive QC assigns column identification number as given below.
- The first column ID shall be assign as
- QLC0001 and second column ID shall be assigned BQLC0002, and so on.
- where
- Q stands for Quality Control,
- L stands for Liquid chromatography
- C stands for Column and
- 0001 stands for the serial number.
- HPLC columns shall be issued and used dedicated for a product and for each test.
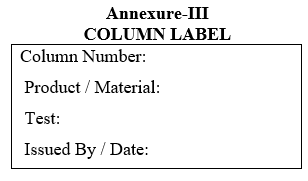
- Label the new column as per Format-III.
- If the GC/HPLC Column fails in system suitability during analysis or physically damaged then Reject the Column and make entry in respective Column usage log, Column Issuance and Rejection Register and Issue new Column for the product or test.
- If rejected HPLC Column passes in system suitability parameters of other product then issue the Column with new identification number. Then Column shall be issued for that particular product or test with format.
- Procedure for maintenance of HPLC Column:
- Before analysis, condition the HPLC column with appropriate ratio of organic solvent and water.
- For reverse phase HPLC Column, after analysis, if mobile phase is used as buffer, then flush the HPLC Column first with water then store the HPLC Column in appropriate organic solvent.
- Fill in the Column usage log after each use in the Format.
- Store the HPLC column with appropriate solvent.
- HPLC column shall be fitted tightly with end caps after analysis.
- Store the HPLC Column in the appropriate HPLC Column box.
- Handle the column with care. Don’t drop or shake the column.
- Procedure for Issuance and In-house numbering of the GC Column:
- GC column shall be issued after receiving the filled request form as per Format.
- Column number shall be assigned as follows. All GC columns shall be identified by “QGC” as the first letter followed by a serial number which shall be of three characters.
For ex.: QGC0001, QGC0002 and so on.
- If the GC column is issued for multiple products then individual system suitability shall be achieved.
- If the GC Column physically damaged then reject the Column and make entry in respective Column usage log and issue new Column for the product or test.
- Fill the Column usage log after each use as per Format.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
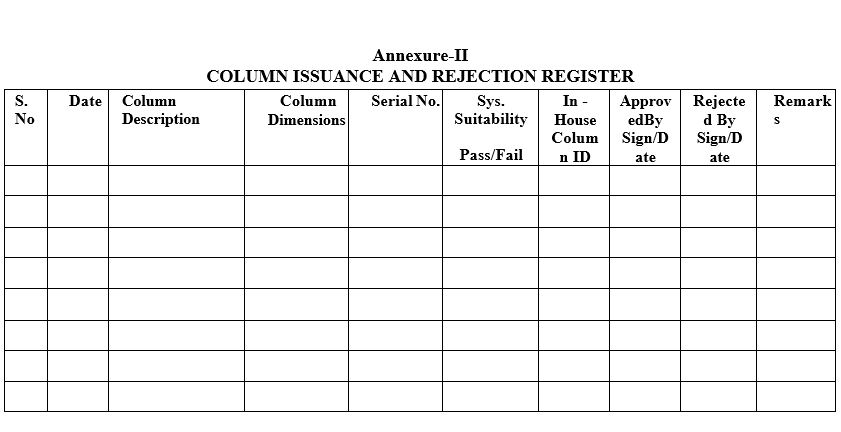
| Annexure-I | Column Issuance/Rejection Request |
| Annexure-II | Column Issuance and Rejection Register |
| Annexure-III | Column Label |
| Annexure-IV | Column Usage Logbook |
Annexure-I
COLUMN ISSUANCE / REJECTION REQUEST
Annexure-II
COLUMN ISSUANCE AND REJECTION REGISTER
Annexure-III
COLUMN LABEL
Annexure-IV
COLUMN USAGE LOGBOOK