
OBJECTIVE:
To lay down a procedure for growth promotion properties of microbiological media.
SCOPE:
This SOP is applicable for growth promotion properties of microbiological media at {Company Name} {Location}.
RESPONSIBILITY:
In charge-Microbiology/ Head-Quality Control: for review and approval of SOP.
Microbiologist- to follow the procedure as per SOP.
ACCOUNTABILITY:
QA Head shall be Accountable for implementation of SOP.
PROCEDURE:
DEFINITIONS The definitions listed here apply in the context of this SOP and may or may not be applicable in all other usage.
Culture – The growth of organisms such as bacteria and fungi in a nutrient medium, usually under controlled condition.
Microbiological media – A nutritive substance, such as an agar gel or liquid medium, in which cultures of bacteria, fungi are cultivated for microbiological purpose
Principle:
Growth promotion properties of the media is one of the most important quality test which gives assurance to the microbiological culture media intended to use in microbiological testing. Improper media formulation or preparation may cause unsatisfactory conditions for microbial growth or recovery and unreliable results.
Growth promotion properties ensures the quality of microbial media by testing each incoming lot of dehydrated media and each sterilized lot of media for their ability for growth promoting, indicative and inhibitory tests using array of specified microorganisms before use.
The microorganisms for growth promotion test shall be selected from the harmonized compendial test chapters for compendial media. For non-compendial media, manufacturer’s recommendation shall be followed. In addition, representative in-house flora may be included as applicable.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/growth-promotion-properties-of-microbiological-media/
Growth Promotion Test requirements:
Growth Promotion test shall be performed for each lot of newly received ready to use media, dehydrated media and each prepared lot of sterilized medium or non-autoclavable medium.
The incoming media lot shall be used for routine testing, only after satisfactory result of growth promotion test.
For diluents, solvents and rinsing solutions, pH and sterility after sterilization should be ensured. Test for growth promotion properties may not be performed. The Growth promotion test shall be performed in dedicated culture handling area having appropriate bio-safety level.
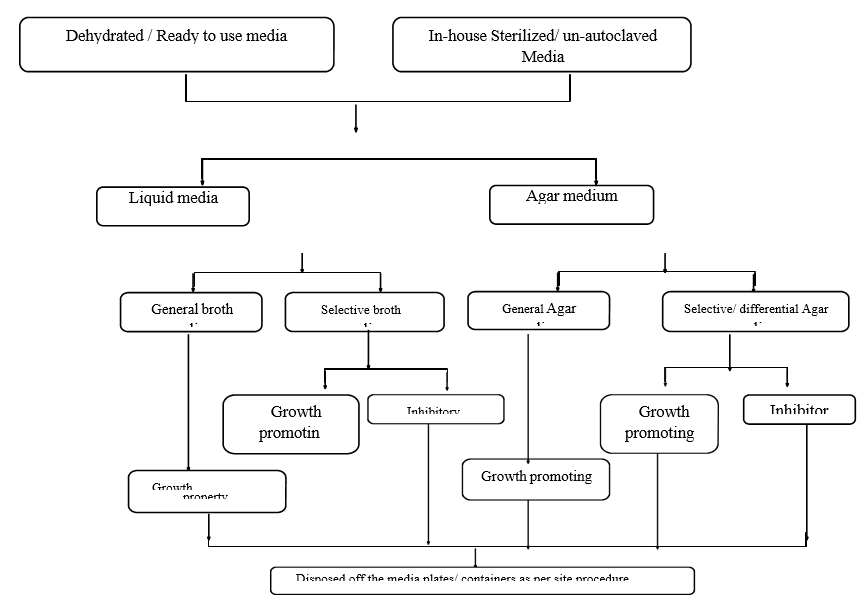
Growth Promoting properties of Solid Media:
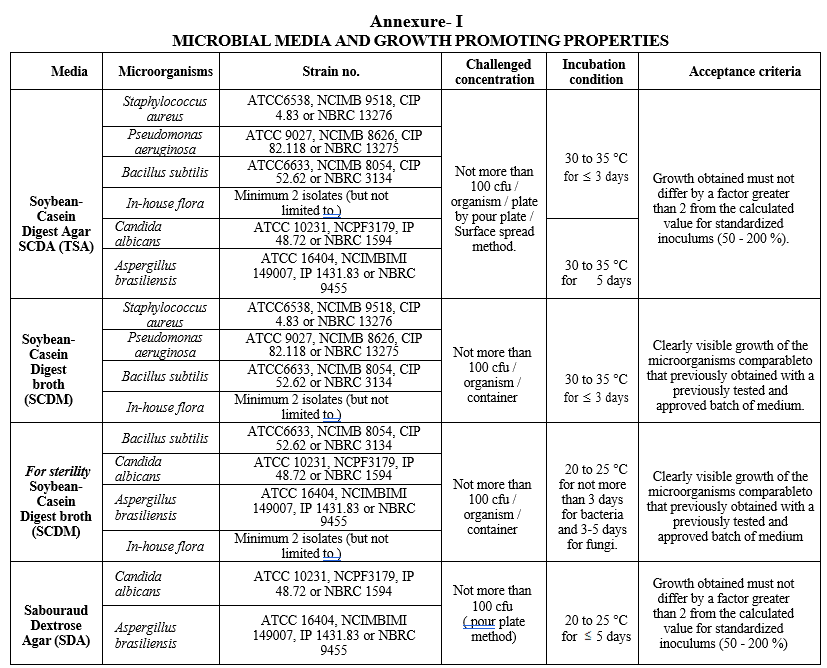
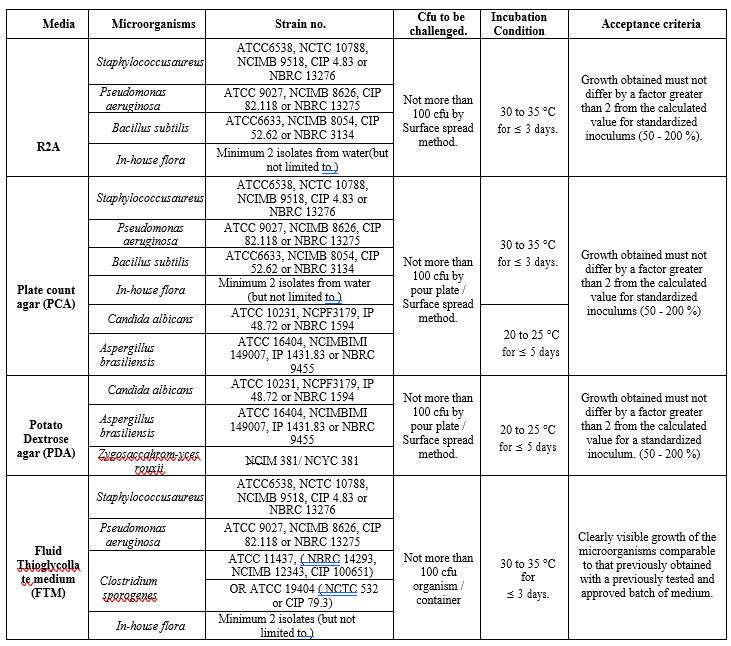
The growth promoting test for each lot of sterilized media shall be performed as per annexure-I.
In case of general solid media, a separate portion/plates of media shall be inoculated with not more than 100 cfu of each specified microorganism using pour plate/surface spread method.
In case of selective- differential solid media, each plate of media shall be inoculated with not more than 100 cfu of each specified microorganism using surface spread method.
Inoculated portion/plates shall be incubated at specified temperature for not more than the shortest incubation period specified in the test. Incubation start and end time should be recorded.
Plates inoculated with fungal culture shall be sealed with parafilm and incubated.
In case of selective- differential solid media the observation shall made at shortest incubation period for growth and further the media shall be incubated for indicative property (characteristic growth) for specified period.
In case of pour plate media used for Microbial enumeration test, parallel growth promotion test shall be performed. However, the Microbial enumeration test shall be released only after the satisfactory growth promotion test result.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/growth-promotion-properties-of-microbiological-media/
Acceptance Criteria:
General solid Media Growth should be obtained within the specified duration and the number of cfu recovered should not differ by a factor greater than 2 from the calculated value of the standardized inoculums (recovery with 50 to 200%).
Selective and Differential solid Media Growth should be obtained within Minimum incubation period and the characteristic growth should be obtained within the specified period of time within the range specified in the test.
Growth Promoting properties of Liquid Media:
Growth promoting test for each lot of sterilized liquid medium shall be performed as per annexure-I.
In case of general purpose liquid medium, each separate portion of sterilized medium shall be inoculated with not more than 100 cfu of each specified microorganism and incubated at specified temperature for not more than the shortest period of time specified in the test. Incubation start and end time should be recorded.
After incubation, the media portion shall be observed for visible growth.
Acceptance Criteria- The clearly visible (turbid) growth of the microorganisms should be obtained within the specified duration (comparable to that previously obtained with a previously tested and approved batch of medium).
Inhibitory Properties of Liquid or solid (Selective/Differential):
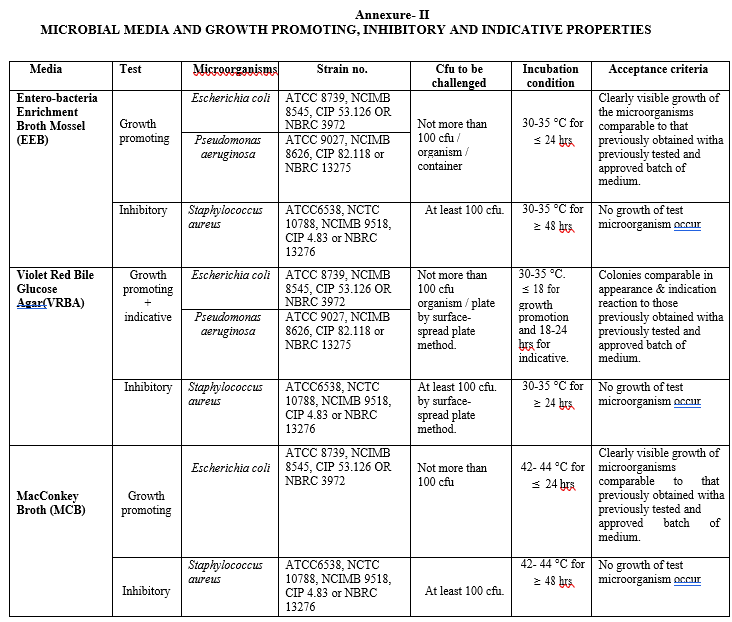
Media Test for inhibitory properties for each autoclaved or non-autoclaved media lot shall be performed as per Annexure-II.
Separate portion/plates of selective/differential medium shall be inoculated with at least 100 cfu of each specified microorganism (appropriate volume of one dilution prior to dilution containing not more than100 CFU/ml shall be used).
The medium shall be incubated at specified temperature for not less than the longest incubation period as specified in the test. Incubation start and end time should be recorded.
After incubation, the media portion/plates shall be observed for growth.
Acceptance Criteria- No growth of specified microorganism should occur.
Indicative Property of solid (Selective / Differential):
Media Test for indicative properties for each autoclaved or non-autoclaved media lot shall be performed as per Annexure-II.
Separate portion/plates of the selective/differential media shall be inoculated with not more than 100 cfu of each specified organism using surface- spread method.
The inoculated portion shall be incubated at a specified temperature for a period of time within the range specified in the test.
After incubation the media portion/plates shall be observed for characteristic growth. If the Growth promoting and indicative organism is same, then same portion/plate shall be used for both the tests. After observing recovery of growth promotion test, same portion/plate of media shall be kept for further incubation for indicative property.
Acceptance Criteria- Growth of microorganism comparable in appearance and indication reactions to that previously obtained with a previously tested and approved batch of medium obtained within the specified duration (time within the range, specified in the test).
A negative control should be performed for each medium during growth promotion test and it should show no growth until the maximum period of incubation.
Media should be used only after the results of growth promotion, indicative and inhibitory properties of the tested media are found satisfactory.
In case of non conformance to the acceptance criteria, an investigation shall be carried out.
The results of analysis performed using particular media should be reviewed and necessary CAPA shall be initiated as appropriate.
In case of laboratory error, observed during investigation, the test shall be repeated for the particular media and compliance shall be re-confirmed.
During investigation, if no laboratory error is observed, necessary actions shall be initiated and appropriate CAPA shall be taken.
For investigation details SOP shall be referred.
After completion of test, the media containers/ plates shall be decontaminated and disposed off as per site specific procedures.
In case of any media or culture spillage, respective SOP shall be followed for handling such incidences.
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/growth-promotion-properties-of-microbiological-media/
FLOW CHART FOR GROWTH PROMOTION PROPERTIES OF MICROBIOLOGICAL MEDIA
REFERENCES:
Not Applicable
ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Microbial media and growth promoting properties |
| Annexure-II | Microbial media and growth promoting, inhibitory and indicative properties |
DISTRIBUTION:
Controlled Copy No. 01 : Head Quality Assurance
Controlled Copy No. 02 : Head QC (Micro)
Master Copy : Quality Assurance Department
ABBREVIATIONS:
| QC | : | Quality control |
| No. | : | Number |
| SOP | : | Standard Operating Procedure |
REVISION HISTORY:
CHANGE HISTORY LOG
| Revision No. | Details of Changes | Reason for Change | Effective Date |
| 00 | New SOP | Not Applicable | To Be Written Manual |
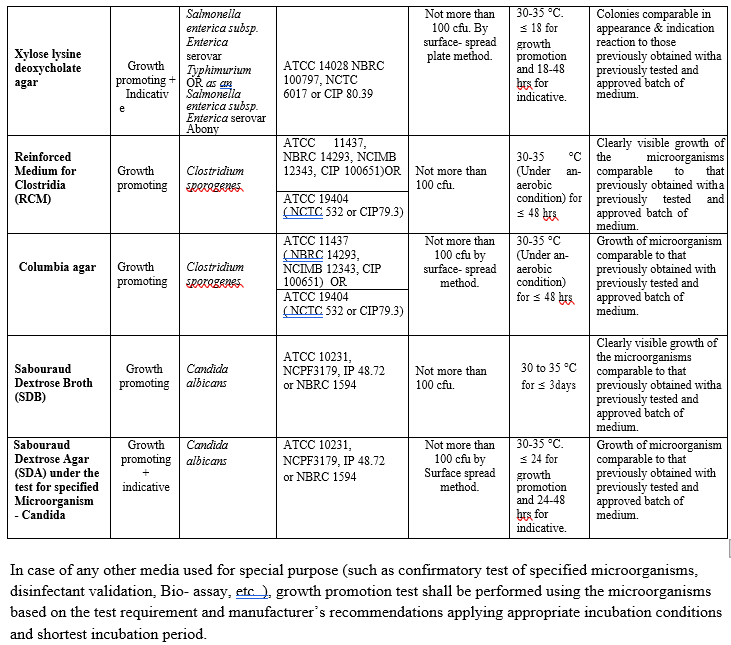
ANNEXURE I
MICROBIAL MEDIA AND GROWTH PROMOTING PROPERTIES
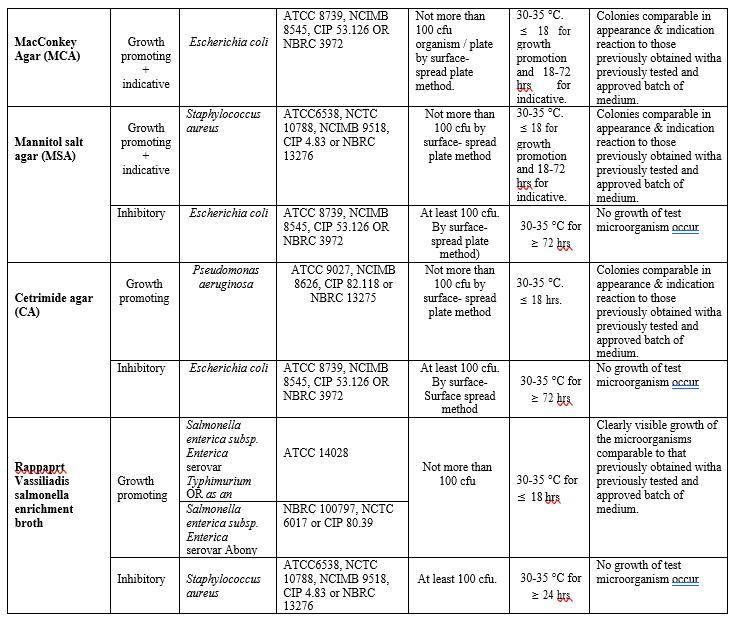
Annexure- II
MICROBIAL MEDIA AND GROWTH PROMOTING, INHIBITORY AND INDICATIVE PROPERTIES
Click the link for download word file copy of this document: https://pharmaguidehub.com/product/growth-promotion-properties-of-microbiological-media/

