- PROCEDURE FOR THE GROWTH PROMOTION TEST:

- A growth promotion test is a quality control procedure used in microbiology. It is a relatively simple test, where the medium is challenged with a small number of microorganisms to assure its nutritive properties and to demonstrate that it enables organism growth.
- Each dehydrated / ready prepared Media, which is used for microbiological testing should be subjected for Growth Promotion test.
- Each lot of received medium should be tested for growth promotion as per GTP.Assign GPT Reference Number to each media undergoing for Growth Promotion Test.
- Assigning GPT Reference Number to Media:
- All the media undergoing Growth promotion test shall be identified with unique numbering system.
- The numbering system consists of 14 characters.
- First three characters “GPT” denote Growth Promotion Test.
- Next character is a “/” slash.
- Next three characters denote Name of the Media undergoing GPT.
- Next character is a “/” slash.
- Next three characters are the serial number starting from 001 in every year.
- Next character is a “/” slash.
- Next two characters “25” denote year 2025.
- Example: The first Soyabean Casein digest Agar medium undergoing growth promotion test in the year of 2022 shall be numbered as GPT/SCD/001/25.
- Procedure for Growth promotion Test:
- Perform the Growth promotion test as per the GTP.
- Record the results of growth promotion in Format.
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/growth-promotion-test/
- In case of failure in GPT for the media, following action shall be taken:
- If GPT fails fewer than two organisms, re-inoculation with the fresh culture shall be carried out and investigation shall includes Conditions of culture preparation & storage, Conditions of media preparation and Conditions of media sterilization.
- For any batch of agar that fails to meet GPT criteria on first test and on re-inoculation PNC shall be initiated and investigation shall be performed. During investigation review (but not limited to)- conditions of culture preparation & storage, Conditions of media preparation, Conditions of media sterilization, Other possibly related GPT Incidents and Data obtained in testing using the media in question.
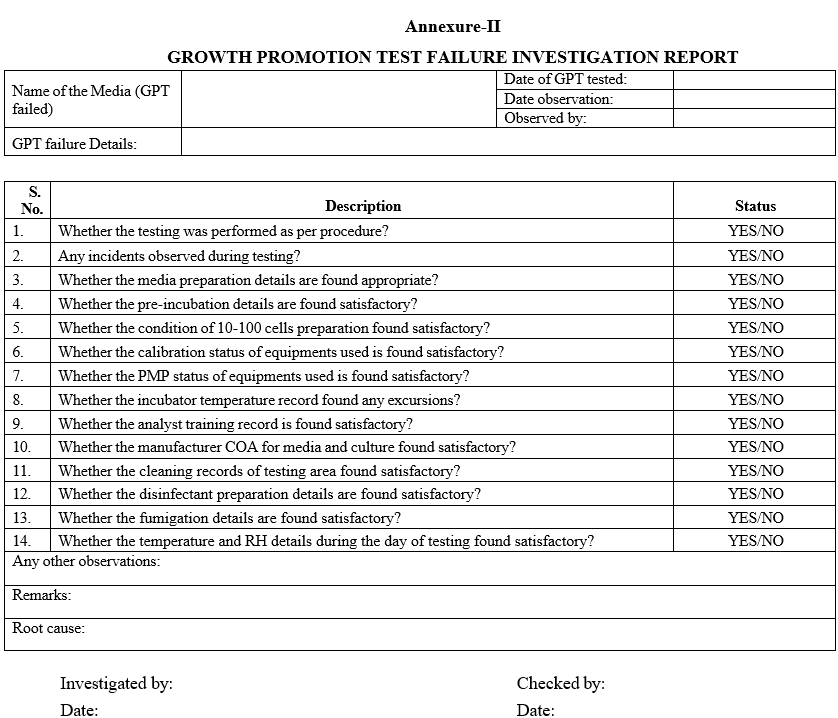
- Investigate the GPT failure as per Format-II.
- Based on the investigation necessary corrective and preventive action (CAPA) shall be taken.
- If the media shows satisfactory growth then the media shall be used for routine analysis.
- ANNEXURES:
| ANNEXURE NO. | TITLE OF ANNEXURE |
| Annexure-I | Growth promotion test reference number allocation register |
| Annexure-II | Growth promotion test failure investigation report |
- ABBREVIATIONS:
| No. | : | Number |
| SCD | : | Soyabean Casein Digest Agar. |
| SOP | : | Standard Operating Procedure |
| QC | : | Quality Control |
Annexure-I
GROWTH PROMOTION TEST REFERENCE NUMBER ALLOCATION REGISTER
Annexure-II
GROWTH PROMOTION TEST FAILURE INVESTIGATION REPORT
Click the link for download word file copy of this document:
https://pharmaguidehub.com/product/growth-promotion-test/

