Status Labeling is a stage where different types of Labels are affixed at various stages of Operations on In Process Containers, Samples, Areas, and Equipments / Instruments. Different Color code is given to various Labels for proper identification.
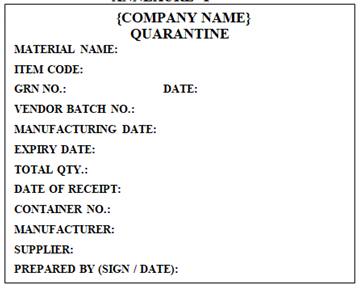
‘QUARANTINE’ Label shall be affixed on thereceived Raw and Packaging Material in Warehouse by Warehouse person
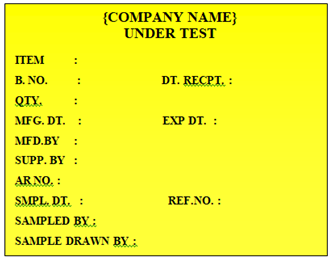
UNDER TEST LABEL: After sampling from the consignment QC Officer shall sample and affix ‘UNDER TEST’ Labelon the ‘QUARANTINE LABEL’, by overlapping the part where ‘QUARANTINE’ word is written
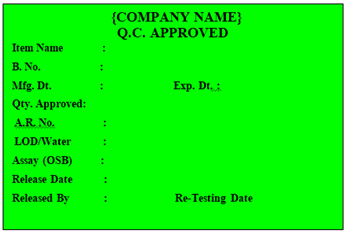
‘Q.C. APPROVED’ Label shall be affixed on theRaw and Packaging Material approved by QC, by overlapping the Under Test Label.
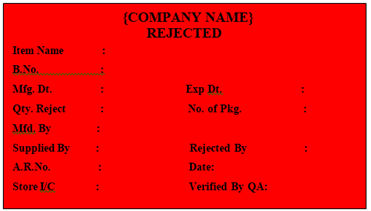
‘REJECTED’ Label shall be affixed on theRaw and Packaging Material Rejected by QC.
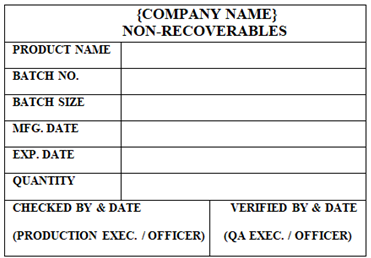
NON-RECOVERABLES’ Status Label shall be affixed on the container which contain materials generated during Manufacturing and Packing activities and cannot be reuse.
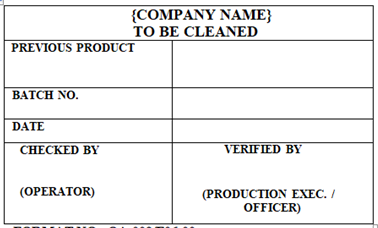
‘TO BE CLEANED’ Label shall be affixed on the IP Containers, Equipments, Utensils, Machines and areas which are to be cleaned.
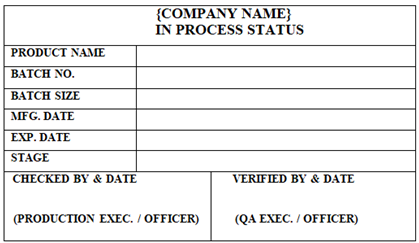
‘INPROCESS STATUS’ Label shall be affixed on the Containers / Equipment which contains Semi- Finished or Finished Materials during operations.
Click the link for complete procedure of Labeling:
https://pharmaguidehub.com/status-labeling/
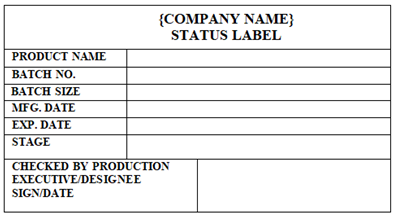
‘STATUS LABEL’ shall be affixed for showing the status of Equipments, IPCs, Machines, Materials Status and Rooms / Areas status etc.
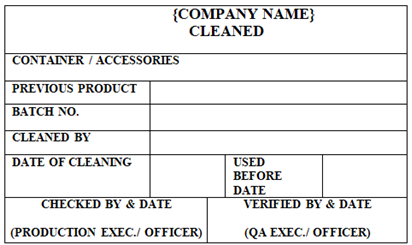
CLEANED’ Status Label shall be affixed on the Containers, Equipments, Utensils, Machines, IPCs and Areas to indicate status of cleaning.
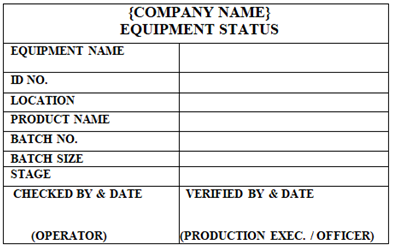
EQUIPMENT STATUS’ Label shall be affixed on the machine during operation time of the machine to indicate the stage of product.
‘LOOSE BOX’ Status Label shall be affixed on the box which contains loose Semi Finished or Finished Materials.

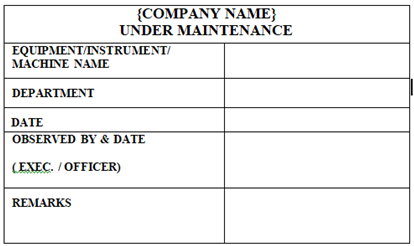
‘UNDER MAINTENANCE’ Status Label shall be affixed on Machines / Equipments / Instruments which are under maintenance.
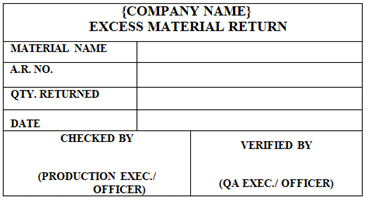
‘EXCESS MATERIAL RETURN’ Status Label shall be affixed on the unused material which is excess after completion of the process (In case of Packaging Material only).
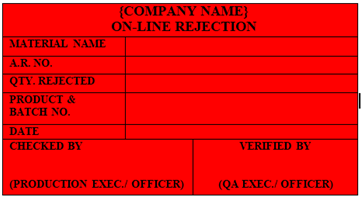
‘ON-LINE REJECTION’ Status Label shall be affixed on the material which is rejected during the Process / Packing Activity.
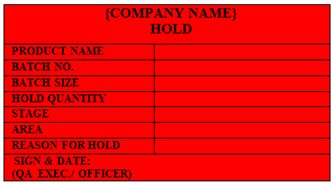
‘HOLD’ Status Label shall be affixed on the Material (Raw material / Chemical / Reagent / Packaging Material), Semi-Finished / Finished Product which is hold due to some reason for further investigation.
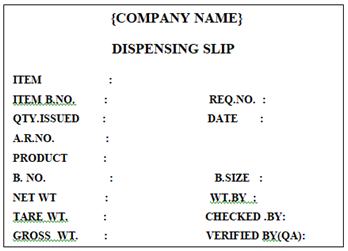
‘DISPENSING SLIP’ Label shall be affixed on the Dispensed Raw & Packaging Material Containers / Poly Bags in Warehouse by Warehouse Person.
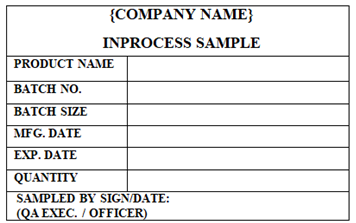
‘INPROCESS SAMPLE’ Label shall be affixed on Semi-Finished Sampled material which is for QC testing.
Click the link for complete procedure of Labeling:
https://pharmaguidehub.com/status-labeling/
‘UNDER TEST’ Status Label shall be affixed on In-process testing material which is under testing.
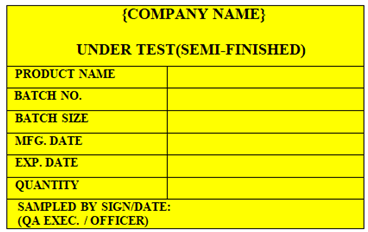
‘APPROVED (SEMI-FINISHED)’ Status Label shall be affixed on the In-process Semi Finished material which is approved by QC.

‘REJECTED (SEMI-FINISHED)’ Status Label shall be affixed on the in process Semi Finished material which is rejected by QA. For Example: Bulk sample fail by QC due to assay problem.

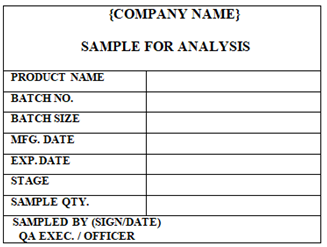
At the time of In Process and Semi-finished & Packing stage QA officer affix the label of SAMPLE FOR ANALYSIS on the Sample bag in which sample is taking for testing.
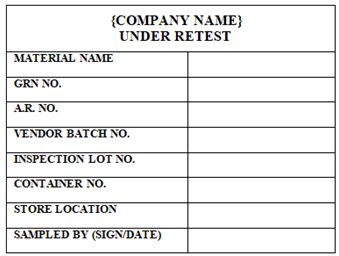
After sampling from the Quarantine Retest Material QC Officer shall sample and affix ‘UNDER RETEST’ Labelon the ‘QUARANTINE RETEST LABEL’, by overlapping the part where ‘QUARANTINE RETEST’ word is written.
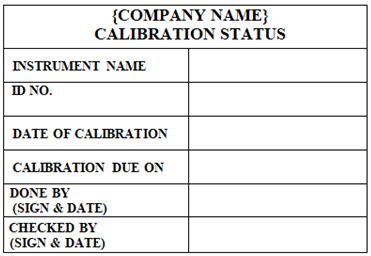
‘CALIBRATION STATUS’ Status Label shall be affixed on the instruments which are calibrated.
Click the link for complete procedure of Labeling:
https://pharmaguidehub.com/status-labeling/